Executive Summary
Across Canada, among all respondents there was a concern regarding the transmission of influenza viruses among host species and recognition of the bidirectional transmission of viruses between humans and swine. Though the likelihood of a pandemic novel influenza virus arising at the swine-human interface is very low, pH1N1 demonstrated that it is possible for such a virus to emerge on the American continent. Novel influenza A viruses can occur through reassortant events occurring in cells simultaneously infected with more than one strain of influenza A virus. Prevention of a reassortant event that could lead to a pandemic novel influenza virus at the swine-human interface requires the prevention of direct and indirect contact between influenza infected persons and pigs. Vaccination of persons in contact with swine and strong biosecurity measures were the top two recommended measures to prevent cross species transmission and the opportunity for a reassortant event. In practice, there are challenges and limitations to the implementation of all recommended measures to prevent cross species transmission of influenza viruses. However, efforts are already underway to meet these challenges. All respondents recognized the need for further efforts that will require the support of public health authorities, government ministries, and the swine industry.
Since the emergence of the 2009 pandemic H1N1 influenza A virus, surveillance has been enhanced across the country in humans through the FluWatch program and in swine through various provincial programs. At the national level the Canadian Animal Health Surveillance Network can readily link and communicate with public health for purposes of surveillance and alerts through the Public Health Agency of Canada (PHAC). Animal disease surveillance is dependent upon submissions from producers generally through their veterinarians. Immediately following the pH1N1 outbreak, as a result of the swine industry experience of public suspicion of pork products and depressed sales, swine influenza A testing in most provinces declined. The cost of testing and concerns regarding confidentiality were identified as the top two challenges for increased animal submissions for influenza testing. In practice, laboratory submissions reflect more closely the degree of uncertainty about a particular disease phenomenon than its incidence. The swine industry is heavily dependent on export markets and recognizes the value of a robust and transparent disease surveillance program. The newly formed Canadian Swine Health Intelligence Network (CSHIN), a national swine surveillance program initiated by the industry, aims to enhance the robustness and transparency of swine disease surveillance through an expert network and complementary practitioner based syndromic surveillance. This initiative is, in part, modeled on successful programs of swine surveillance in Quebec and Alberta. With its expert network, CSHIN can be more sensitive in detecting disease trends than passive laboratory surveillance alone. All respondents agreed that the response to influenza, regardless of its zoonotic pandemic potential, needs to be appropriate and commensurate with the risk in the respective species. Government response needs to be carefully planned and coordinated. Clear public messaging is important. The capacity for zoonotic influenza research in Canada is strong but funding is a very real limiting factor.
Acronyms
APHIS – Animal and Plant Health Inspection Services, United States Department of Agriculture
BMP – Best management practices
CFIA – Canadian Food Inspection Agency
CAHSN – Canadian Animal Health Surveillance Network
CDC – Centers for Disease Control (USA)
CIHR – Canadian Institutes of Health Research
CMOH – Chief Medical Officer of Health (provincial)
CSHB – Canadian Swine Health Board
CSHIN – Canadian Swine Health Intelligence Network
CVO – Chief Veterinary Officer
CCVO – Council of Chief Veterinary Officers
HPAI – highly pathogenic avian influenza
ILI – influenza-like-illness
MAPAQ – Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec
NACI – National Advisory Committee on Immunization
NCFAD –National Centre for Foreign Animal Disease
NML – National Microbiology Laboratory
OH&S – Occupational Health and Safety
OIE – World Organization for Animal Health
pH1N1 – pandemic H1N1 influenza A strain of 2009
PHAC – Public Health Agency of Canada
PPE – personal protective equipment
PRRS – Porcine Reproductive & Respiratory Syndrome
SEAC – Surveillance/Epidemiology Advisory Committee
VS – Veterinary Services of the APHIS
Introduction
Influenza virus infection in all animals can lead to a contagious respiratory illness. In humans or pigs the illness can range from subclinical to severe, including death, depending on strain virulence and host factors. People with certain preexisting conditions are at higher risk for serious complications from infection with influenza (http://www.cdc.gov/flu/about/disease/). In both species influenza infection causes varying degrees of fever, coughing, sneezing, and myalgia. Transmission is primarily through close proximity and the inhalation of infected aerosolized droplets or infected dust particles, or indirectly following contact of infected surfaces (CDC).
Influenza in pigs was first recognized clinically in North America during the 1918 Spanish influenza pandemic. From 1930 when the first influenza virus was isolated in swine until 1998 swine influenza viruses in North America consisted of a predominant subtype known as classical swine influenza (cH1N1) which consisted primarily of swine influenza genes. The segmented RNA genome of influenza A viruses allows for genetic material reassortment between viruses co-infecting the same host leading to the generation of new antigenically novel viruses. Since 1998, a triple reassortant H3N2 influenza virus (trH3N2) composed of swine, avian, and human genes emerged in global swine populations. As further reassortant viruses have emerged in swine populations, the predominant endemic subtypes in North America consist of trH1N1, trH3N2, and trH1N2. In addition, a trH3N1, a wholly human H1N1, and wholly avian H3 and H1N1 influenza viruses have been isolated from swine in North America (Thacker and Janke, 2008). The pH1N1 virus is believed to be in wide circulation among swine globally (Nelson, 2012). Evolution of swine influenza viruses through reassortment, known as genetic shift, is expected to continue.
Transmission of influenza viruses among host species is a concern and bidirectional transmission of influenza viruses between humans and swine is well recognized. Higher than average levels of antibodies to swine influenza viruses have been documented among swine workers (Gray et al., 2007; Myers et al., 2006); however, cases of human illness caused by swine influenza have been rare. Sporadic human infections with each of the 3 major endemic swine influenza A subtypes have been documented in the USA. Previously, the CDC reported approximately one case of human infection with a swine influenza virus every one to two years (http://www.ars.usda.gov/2009h1n1/). These viruses in humans are referred to as variant viruses denoted by the letter “v” (e.g., H1N1v, H3N2v and H1N2v). The vast majority of human infections with variant influenza viruses do not result in person-to-person spread (CDC). In 2010, an H3N2 swine virus containing the matrix (M) gene from the 2009 pH1N1 virus was first isolated from swine in North America. In 2011, a specific trH3N2v virus with 2009 H1N1 pandemic virus M gene was isolated in humans associated with swine contact in the USA (CDC). The acquisition of the 2009 M gene may contribute to enhanced transmissibility of swine influenza viruses to humans atypical for other swine influenza viruses (CDC).
Interspecies transmission of influenza virus between humans and swine, though well documented, has not resulted in markedly virulent disease in either species. However, though the influenza pandemics of the past have been associated with the introduction of avian influenza viruses into human populations directly (Thacker and Janke, 2008), the pH1N1 virus pandemic demonstrated the real possibility of a novel pandemic influenza virus arising at the swine-human interface.
In a one-health approach to influenza virus infections at the swine-human interface, it is incumbent upon Canada to protect both human and swine populations from interspecies transmission and furthermore, to guard against, and detect as early as possible any novel potentially pandemic strain.
The purpose of this project was to gather expert opinion on the current state of influenza prevention, detection, and mitigation of zoonotic influenza at the swine-human interface. Opinions were sought regarding the best means for the swine industry and all levels of government to work together to prevent and detect a potentially pandemic zoonotic influenza virus in Canada.
Methods
Throughout the fall of 2012, expert interviews were conducted or questionnaire responses were collected among the following groups of experts within Canada:
- Virologists with expertise in influenza of human and/or swine origin
- Swine health academics with expertise in swine influenza
- Influenza researchers with expertise in influenza epidemiology
- Provincial Chief Veterinary Officers or their delegates in key swine producing provinces
- Canadian Food Inspection Agency scientists
- Non-governmental Swine Health groups and representatives from the swine industry
- Provincial Chief Medical Officers of Health or their delegates
Interviewees were sent an interview transcript for their review, based upon a digital recording of the interview or an interview summary based upon notes, including verbatim quotations, taken during the interview. All respondents were asked if they could be listed among the respondents and if they would like a copy of the report.
In this report, non-attributed participant quotations are presented in italics within text boxes.
Findings
1. Participant general comments
The human and animal health sectors both have a complex multi-layered infrastructure for addressing health concerns. It is important for the stakeholders to understand each other’s responsibilities and how the health care system for humans differs from that of animals. A one-health approach requires a deep understanding of the health systems on both sides of the human-animal interface. Canadian veterinarians are concerned about the potential risk of a novel influenza virus arising at the swine-human interface, although the risk from South East Asia where intensive contact among people, poultry, and pigs occurs is perceived to be much greater.
2. The swine industry in Canada.
Care must be taken in any surveillance strategy to avoid the appearance that swine and pork production are to blame for the emergence of pandemic influenza. There is not enough evidence to support this view, but some public health documents leave this impression. This perception could put an industry out of business.

Domestic consumption of pork accounts for only 30% of production in Canada. The Canadian swine industry enjoys a world-wide reputation for a safe, high quality pork product and for high quality breeding stock that is shipped to over 50 countries worldwide. Canada’s pork industry is a top exporter due in part to its healthy herd. Since 1995, Canada’s pork export market grew considerably; in 2011, the swine export market was worth 3.2 billion dollars. Currently, the USA and Japan account for approximately half of the total export market. The export market for swine genetics is smaller than the meat market but important nonetheless. Products such as live animals, embryos, and semen already have requirements for quarantine and specific testing.
The industry success has resulted from the collaboration among genetic suppliers, researchers, national organizations, and governments. The swine industry in Canada produces swine that are recognized to be free of 10 OIE-listed reportable swine diseases including the zoonotic diseases trichinella and anthrax. There are national on-farm food safety and animal care programs such as the Canadian Quality Assurance Program for Canadian Hog Producers (http://www.cqa-aqc.ca/about-e.php) and Animal Care Program (http://www.cqa-aqc.ca/aca/index-e.php) respectively.
“The swine industry is complex, sophisticated, and tightly controlled”.
“State-of-the–art hog production entails highly trained persons, familiar with herd health, animal care, and quality assurance programs.”
Many operations specialize in one portion of swine production such as the production of grower pigs on breeder farms or the finishing of these growers. Many farms that raise growing animals to market weight move groups of animals through the finishing period together to control disease (all-in, allout). Many farms follow strict biosecurity measures such as down-time requirements for staff between contact with swine at other farms and shower-in entry, particularly on breeder farms. The herd veterinarian is the first line of defense in monitoring and surveillance. All Canadian swine farms have access to a herd health veterinarian who is supported by a nationwide network of veterinarians and diagnostic laboratories.
“Canadian veterinarians are well organized and work together on national matters.”
Capacity for research through universities and swine centres is excellent and supported by producer associations. National organizations set research priorities for the industry. The Canadian Swine Health Board (CSHB), a multidisciplinary group of representatives from the Canadian Association of Swine Veterinarians, Veterinary Colleges, Canadian Centre for Swine Improvement, Canadian Meat Council, Canadian Pork Council, and Council of Chief Veterinary Officers provides leadership, coordination and support in the management of the health of the Canadian swine herd. The CSHB works closely with CFIA, Agriculture and Agri-Food Canada, and provincial Ministries of Agriculture. The CSHB has developed the National Swine Farm-Level Biosecurity Standard, clear research priorities, and plans for long term disease risk management that includes leadership in addressing potential zoonotic threats. As such, the CSHB has been the industry voice in terms of One Health matters, and has worked in collaboration with PHAC on several One Health initiatives.
A long term goal of the industry is to increase export markets. Export markets are strengthened through trust and transparency. Currently, producer countries with the maximum number of specific disease-free designations enjoy the widest possible export markets. However, with the recognition that disease status in a national herd is somewhat dependent upon the degree of testing for the specific disease in question, the OIE is moving towards a system in which a country is not only declared free of specific diseases through periodic monitoring but also rated on the robustness of their disease surveillance system. Denmark, a world leader in comprehensive and transparent surveillance, enjoys worldwide confidence in its ability to detect swine diseases in a timely manner. The CSHB has partnered with CFIA to enhance Canada’s capacity to demonstrate freedom from certain diseases in accordance with this new approach by the OIE. Canada’s swine industry also recognizes the benefits of enhanced and transparent surveillance and anticipates that the Canadian Swine Health Intelligence Network (CSHIN) will provide the ability to demonstrate swine health on a near real-time basis.
“Market forces respond very quickly to uncertainty.”
In this age of instant communication, markets can be closed without warning upon receipt of unconfirmed information. The impact on the industry of a novel zoonotic influenza virus in the Canadian swine herd depends upon its virulence in swine or transmissibility to humans and how the discovery is communicated in the media. The impact could range from no impact to a severe impact. Negative public perception regarding food safety can lead to reduced consumption of pork, possible market closures, and a drop in demand for Canadian pork and live swine with negative consequences on pork farmers’ and producers’ “bottom line”. If meat processing plants cannot export to Canada’s major markets or if retailers in Canada anticipate a food safety problem, the number of pigs slaughtered would sharply and immediately decline. Overcrowding and welfare issues can result if producers cannot find markets for their pigs. Some farms would have to resort to mass euthanasia of swine in the short term. In the long term, a reduced price can result in producers having fewer veterinarian visits and doing fewer diagnostic tests leading to reduced laboratory surveillance. In the early stages of the pH1N1, erroneous public perception of swine products as being unsafe affected the industry in all provinces equally regardless of where it was first found in swine. The impact may be felt less intensely if a novel zoonotic influenza virus event occurred outside Canada but since flu spreads rapidly it wouldn’t take long for all Canadian producers to feel the impact.
During the H1N1 pandemic, the industry did benefit from the involvement of the public health infrastructure. The pH1N1 virus was characterized by PHAC through the NML and subsequent coordinated communications and response taken by the CFIA and PHAC at the national level was helpful to the industry. That serious situation became manageable domestically but the industry, with the help of the CFIA, then had to deal with the export trade problems. Markets such as China blocked Canada’s swine products, while other markets imposed restrictions or additional obligations or requirements for the import of Canadian pork. Although it was shown that meat products were safe, it took longer for the export meat market to return to previous levels than for the domestic market.
“The public has to have confidence in the health authorities for there to be trust. This confidence is present in Canada.”
The experience of pH1N1 and research results concerning swine influenza virus (SIV) have raised the level of awareness among swine veterinarians and producers.
“Veterinarians are very aware of the issues around influenza virus.”
Swine veterinarians and their professional organizations encourage their members to get the flu vaccine and similarly encourage their producer clients and producer staff to do the same. Swine veterinarians also instruct their clients to enforce policies prohibiting entry of any persons exhibiting influenza-like-illness (ILI). Nevertheless, swine producers operate in a highly competitive industry and face greater concerns regarding disease challenges than novel zoonotic influenza at the swine-human interface.
3. Influenza virus in humans and pigs, and reassortant viruses
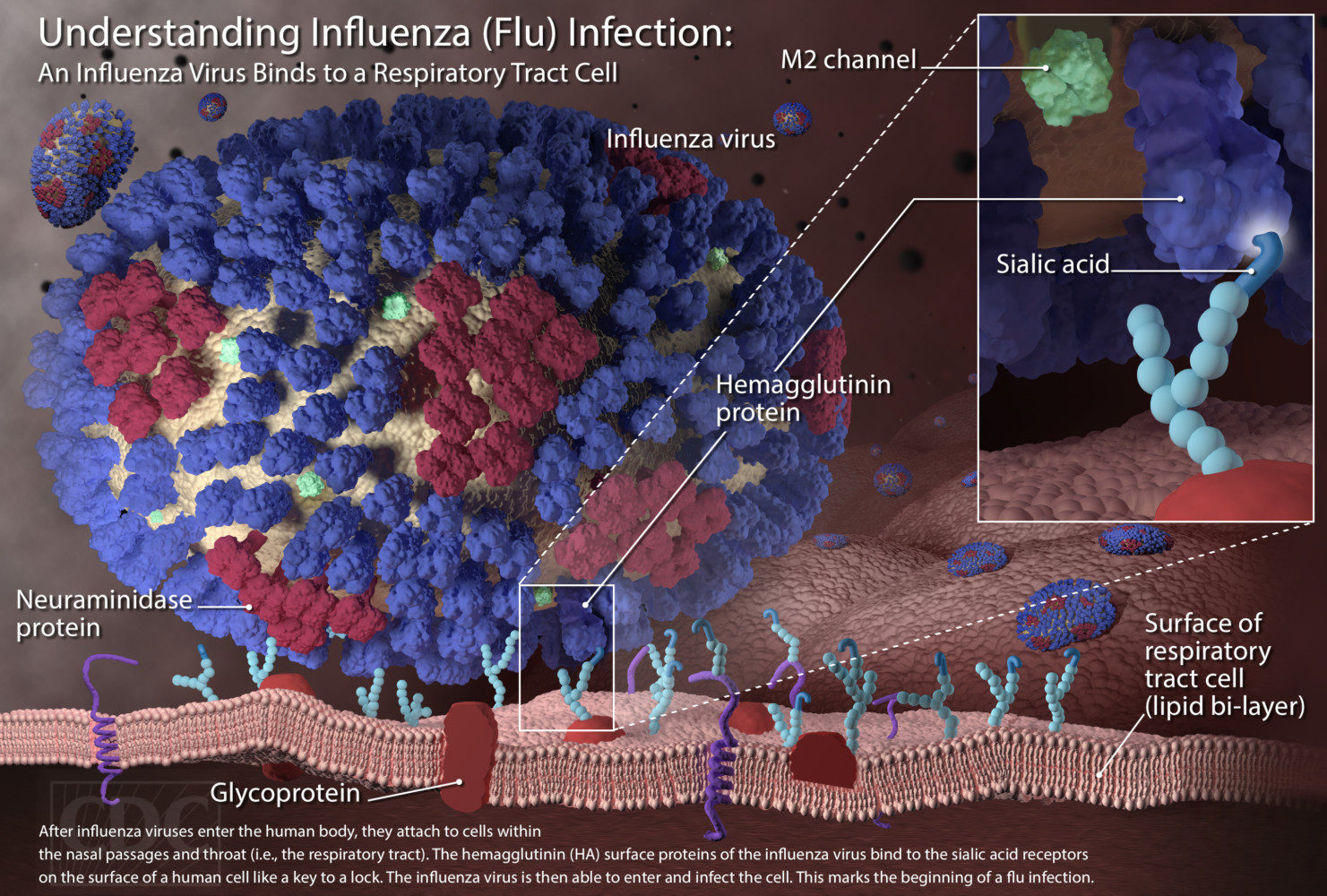
Influenza is not unique to one species, it is a virus that affects many mammalian and avian species. Influenza A, B and C viruses can cause public health problems, however only influenza A viruses infect both humans and pigs. Reports from Japan and China claim that they have detected influenza C in swine. It is unclear to what extent Influenza C has been examined in pigs in other regions. Humans and seals, but not pigs, can be infected by Influenza B. Both humans and swine have their own influenza A strains. It has been demonstrated that both the pig and the human respiratory tract contain both types of sialic acid receptors that will allow infection with human, swine, and avian influenza viruses. Because of reassortment, there has been gene transfer among influenza A strains that have crossed species barriers among human, swine, and avian influenza A viruses.
Given that bidirectional cross-species transmission is recognized and that genetic shift resulting from reassortant events has been documented in swine (Poljak et al., 2004; Liu et al., 2009; Nelson et al., 2012) and a reassortant event in a human between the pH1N1 and seasonal flu viruses (H3N2) has been documented (#42 ProMed report, June 9 2011), it is theoretically possible that a cross species reassortant event could occur in either species. In practice, an event in which a human acted as a cross species reassortant “mixing vessel” has never been documented whereas swine as “mixing vessels” has been recognized.
Genetic drift in swine influenza viruses is believed to occur, but at a much slower rate than for influenza viruses in humans. Swine influenza viruses may experience different evolutionary pressures in their relatively short life-span hosts compared to human influenza viruses. Infection of swine with human influenza strains is well recognized in the swine industry. Some SIV lineages link back to human strains, the swine Influenza AH3N2 for example originated from a human strain that has since diverged and become endemic in pigs. Some human influenza virus strains cross more easily into pigs; common lineage may be the reason. Based on some of the swine surveillance work underway in Canada there are novel SIV strains that contain 2009 pH1N1 genetic material already in circulation in the Canadian herd.
“Many other species are susceptible to influenza virus infections.”
However, it is the level of contact among pigs, birds, and humans that make attention to influenza viruses in swine and birds more important.”
There is a concern for increased risk of novel reassortant influenza where there are opportunities for swine /domestic and wild birds /humans, and perhaps other species regarding influenza, to be in close contact. According to respondents, if multispecies farms raise poultry indoors in barns separate from swine, with separate staff, and prevent children from entering the barns, then the risk may not be that much different from single species farms. Most farmers change clothes and wash hands before and after being in the barns. For multispecies farms with swine, poultry, and waterfowl comingling outdoors, more effort should be made to include these farms in any surveillance.
“More focus in influenza surveillance and prevention should be given to international human travelers that enter swine barns—workers, owners, vets, tradespeople and visitors.”
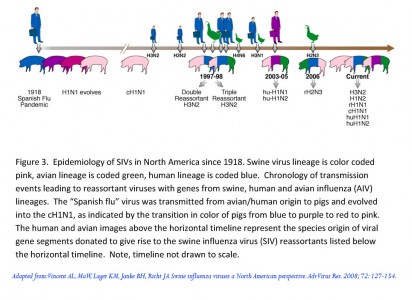
Figure from the USDA SIV website.
Temporary foreign swine workers in Canada are often from the Philippines. They travel back and forth from Asia while they work in pig barns in Canada, making them potential sources of novel viruses. Producers, veterinarians, and tradespeople also travel frequently around the world, entering pig barns or markets as consultants or aid workers in other countries, making them potential vectors of novel viruses. Indeed this is thought to be how pH1N1 came to a Canadian swine farm (Howden et al., 2009).
Prevalence of SIV is likely greater among swine in Canadian provinces with more swine production. In one province with a lot of swine operations, serological surveys suggest that at the animal level approximately 58% of sows and 18% of finished hogs have been exposed (i.e., have antibodies), while at the herd level approximately 83% of sow herds and 40% of finisher herds have been exposed. Porcine Respiratory Disease Complex (PRDC), a term used for respiratory disease of multiple etiologies, can include co-infections with mycoplasma, secondary bacteria, porcine reproductive and respiratory syndrome (PRRS) virus, porcine circovirus type2, and SIV. Porcine Respiratory Disease Complex does have a significant impact on the industry but the exact role of influenza in PRDC is not well understood. In general, swine influenza strains are not highly pathogenic. Sows may experience stillbirths but in grower populations the infection typically causes some coughing and a slight temporary growth rate reduction. Nursery piglets and young growers are the most susceptible populations and may experience deaths. Virus shedding can start within 12 hours of infection followed by typical clinical signs of coughing, sneezing, and respiratory distress by 48 hours post infection. In enzootically infected herds the disease is usually subclinical; typical signs of influenza may occur in only 25% to 30% of the pigs. In a newly infected herd, up to 100% of the animals may become ill, but most animals recover within 3–7 days if there are no secondary bacterial infections or other complications. During this time the pigs may grow more slowly and as a consequence they don’t reach market weight as quickly as expected. While these pigs are held back from shipping to market, barns can become more crowded for a short time which may impact other health or welfare issues. Among swine there is seasonal variation in the incidence of SIV with early spring and late fall being the times of year with the most cases. Vaccinated sows will pass immunity to their piglets. Vaccinating the piglets as they leave the nursery at approximately 12 weeks with a killed influenza vaccine containing the appropriate strains protects them during the grower period.
There is an economic impact of SIV but it is not highly evident to producers. With increased research in the last 4-5 years, production losses associated with SIV are becoming more recognized. The more economically important viral infections in pigs, PRRS and porcine circovirus type2, can occur concurrently with SIV infections. Some Influenza A H3N2 of swine origin can cause a severe and permanent drop in egg production of breeder turkeys; as a consequence, turkey producers consider some SIVs to be very serious.
4. Research capacity
There are capable labs and knowledgeable researchers in Canada. Although Canada has plenty of research expertise in the area of influenza viruses, respondents feel that funding is inadequate to support the necessary research. During the H1N1 pandemic, the Pandemic Preparedness Strategic Research Initiative was launched. This initiative supported by fundingfrom CIHR, CFIA, and PHAC, succeeded in building research capacity in Canada in the area of influenza virus research. This source of funding is now very limited. If research on zoonotic influenza with pandemic potential research requires BSL-3 facilities, Canada is limited to four such labs: Abbotsford Animal Health Lab of British Columbia, Winnipeg-NCFAD, Saskatoon VIDO/INTERVAC, and Guelph-OVC. Increased regulations (e.g., transportation permits) which restrict virus sharing between human and animal laboratories also pose challenges to studying and working with zoonotic influenza viruses. Researcher liability also needs to be addressed. A number of groups have done work on zoonotic influenza; however, this work is usually in response to outbreaks of ILI in swine workers etc., there is no active ongoing research program.
Summary of some zoonotic influenza research and SIV research:
a) The zoonotic flu study in Alberta completed three years of monitoring influenza viruses (2009-2012) at the swine-human interface. This study faced the challenge posed by the pH1N1 outbreak. The adverse publicity surrounding the Alberta farm with pH1N1-infected pigs resulted in a sudden decline in farm willingness to participate. No reassortants were found.
b) The National Centre for Foreign Animal Diseases (NCFAD, CFIA) is doing some ad hoc research and has the capacity for more research if funding became available. If funding were available, other research groups could contribute to research efforts in Canada.
c) SIV research is conducted periodically at Canadian veterinary colleges. There may be greater industry interest in influenza research in pigs if studies investigated SIV production affects.
d) In the USA, Veterinary Medical Officers Amy Vincent and Kelly Larger are leading the USDA Agriculture Research Service’s influenza in swine research (http://www.ars.usda.gov/2009h1n1/).
“There will always be a risk and the pH1N1 shows that a novel reassortant virus can occur in North America.”
“Through good herd health and seasonal vaccination of swine workers, some degree of
prevention may be accomplished.”
5. Prevention of zoonotic influenza and control of influenza in humans and pigs
Novel pandemic influenza in people or animals that causes severe illness and mortality is a recognized threat. However, not all transmission of influenzas between pigs and people can be prevented. A reduction of species-specific influenza A in humans and pigs alike would help reduce the likelihood of a reassortment event occurring at the swine-human interface.
“Prevention of a reassortant event requires the avoidance of co-infection of influenza virus from separate species.”
“Key messages include keeping children out of barns, washing hands, getting vaccinated, and keeping sick people out of barns.”
“Susceptible people (e.g., immuno-suppressed) should avoid close contact with animals and take extra hygiene precautions.”
Key points to prevent a reassortant event:
- Vaccinate swine workers. The CSHB has been proactive in promoting vaccination among swine workers through direct information to producers and by holding flu shot clinics at their meetings (Read Here) Swine veterinarians may be required by their clients to be vaccinated with the flu shot. Seasonal flu vaccination is widely supported by veterinarians and the swine industry itself. In 2012, the CFIA distributed a circular to the swine industry to encourage swine workers to get the seasonal flu vaccine. Several provinces have held education and awareness campaigns targeted at swine workers and Hutterite colonies. In some provinces the Ministry of Health and Ministry of Agriculture collaborate in targeting farm workers (poultry and swine) in promotion of campaigns for seasonal flu vaccination. Vaccination of swine workers should extend to their immediate families.
- Vaccinate swine with influenza vaccine. Producers and swine workers need to better understand how vaccination of swine helps their own health, their financial bottom line (by helping to prevent swine influenza infections, a production-limiting illness), and society (by reducing the probability of cross species influenza virus coinfection and possible zoonotic reassortants).
- Prevent contact between ill swine workers, and pigs through good general biosecurity measures. Implementation of the National Swine Farm-Level Biosecurity Standard that addresses all diseases would be the most effective approach. Biosecurity promotion in the swine industry is often a shared responsibility among the provinces, the CSHB, industry groups, and sometimes the CFIA. http://biosecurity.swinehealth.ca/
In practice, the implementation of each prevention measure faces limitations and challenges:
5.1 Vaccination of swine workers:
- Annual flu vaccines include the following influenza A strains: seasonal H3N2, seasonal H1N1, and pH1N1; the exact strains are selected by a WHO panel. Strains included in the seasonal influenza vaccination do not always exactly match currently circulating strains. A person can become infected and shed virus despite vaccination though the severity of illness is usually reduced. Not all human flu infections can be prevented through annual vaccination.
- Vaccination of all swine workers may be impractical:
i. Some religious groups oppose the principle of vaccinations for themselves, even if they vaccinate their swine (e.g., porcine circovirus).
ii. Vaccination is free in some provinces but not in all. In some provinces where it is not free for all persons, it may be free to population sub-groups such as those with chronic health conditions that place them at high risk of complications from influenza, health care workers, and poultry industry workers.
iii. Swine workers are not among the National priority groups for flu vaccination as identified by the National Advisory Committee on Immunization (NACI), http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/13vol39/acs-dcc-4/assets/pdf/13vol39-acs-dcc4-eng.pdf. However, some provinces add swine workers as an additional priority group eligible for free flu vaccine.
iv. Accessibility of flu vaccination may be an issue. Vaccination uptake may be improved if vaccination is widely accessible. Access to flu clinics in remote areas may be limited. In the more rural provinces distances can be large, and unless flu vaccination is mandatory or subsidized or seen to be a high priority, many will not make the effort to get vaccinated.
v. Young children are at greater risk of infection with influenza as they lack prior immunity and are more likely to transmit the infection through their close contacts with family, friends, and community members. Young children with direct or indirect contact with swine may not be recognized as a priority group.
vi. There are some temporary foreign workers in the Canadian swine industry who may be ineligible for provincial health care insurance plans and who may not utilize the health care system to the same degree as Canadians. Many of these swine workers have limited English skills and are here for only a short time. There may also be cultural barriers to getting vaccinated.
vii. Approximately one third of the Alberta’s pig producers are Hutterites. Though many Hutterite community members are vaccinated for flu through regular public health flu clinics, reaching this community with a targeted education program and with colony visits can be labour intensive.
“Free vaccine alone is not enough to get people vaccinated.”
5.2 Vaccination of swine:
- Currently, all SIV vaccines are killed vaccines. Swine influenza strains constantly evolve, though perhaps not as quickly as human strains; consequently vaccines need to be routinely updated. Swine influenza vaccine manufacturers rely on research done by other researchers; they do not conduct their own surveillance of swine influenza strains. Until recently, vaccine companies have been required to reapply for a new license for each new influenza vaccine formulation. Waiving this requirement is likely to result in more frequently updated swine influenza vaccine formulations. However, private industry does not share proprietary information about the strains included in vaccine formulations. On-going surveillance of SIV strains may help to update vaccine formulations. Autogenous vaccines are becoming more commonly used in the USA and more recently in Canada. A laboratory in Ontario provides this service.
- In Canada, the proportion of vaccinated herds is believed to be far less than in the USA. The actual vaccination rate is unknown and likely varies among the provinces.
- Vaccination of swine generally occurs within the breeder populations, gilts and sows, in particular. Not all breeder operations use SIV vaccination and those that do may only vaccinate seasonally. The cost of vaccination of grower pigs, for what is considered a mild disease, might be regarded as an elective additional cost of production. In tough economic times, producers are particularly sensitive to production costs.
- Reduction of transmission may be accomplished through vaccination but even with vaccination it has proven difficult to eliminate SIV in pig herds.
5.3 Prevention of contact between ill swine workers and pigs:
- As in humans, infected pigs can shed influenza virus 24-48 hours prior to demonstrating clinical signs and subclinical infections can become clinical if the host is stressed. Transmissible infections may go unnoticed in either species. Human adults can remain infective for up to 6 days and children for up to 10 days (CDC). Transmission could occur after the patient has returned to work.
- Smaller farms may not have access to replacement workers, especially on short notice (i.e., in the case of a family farm).
- Swine workers who are foreign nationals may be particularly reluctant to miss work days on account of being unable to afford to miss work days. Not all farms are willing to pay for sick days though some larger farms do pay ill workers. Additional reasons for temporary foreign swine workers to continue working despite an illness includes the fear of jeopardizing their status in a temporary foreign worker program resulting in firing or deportation. Temporary foreign swine workers may be ineligible for provincial health care insurance plan benefits as well as Workers Compensation Program benefits even if they were given a doctor’s note for enough time off work to qualify for the benefits extended to other workers. This is something that should be addressed with industry and provincial Ministries of Labour Relations and Workplace Safety.
- Wearing a face mask or N95 mask could provide protection against transmission of human influenza viruses and is a routinely recommended biosecurity measure but may not be well tolerated while performing physical farm labour.
5.4 General Farm Biosecurity measures & considerations:
- The tightest biosecurity and biocontainment measures are more likely to be implemented on larger farms and integrated production systems. These may include isolation of ill pigs, use of air filters, shower-in shower-out practices, full washing and drying of farm trucks, and prevention of entry by persons in recent contact with other swine.
- Some swine operations are operated by communities (e.g., Hutterites) in which there is a culture of visiting between members of one community with another. Preventing swine worker contact with swine of another farm is more difficult in these situations.
- Location of farms within pig dense areas and in areas with poultry operations, particularly operations with turkeys, make effective biosecurity challenging.
- Swine influenza viruses can usually be transmitted a few meters though airborne means. The farthest distance that viable SIV can be transmitted through airborne means is the subject of research. In one province, it was noted that swine operations located along a major highway experienced a higher incidence of SIV infections than elsewhere. In another province it was noted that swine farms downwind from the index case farm were more likely to be affected by the SIV outbreak. The extent to which air filters may prevent or contain airborne transmission is unknown.
- Fomites can also act as indirect transmitters of influenza virus making prevention of transmission more difficult.
- Some farms such as Hutterite, Mennonite, and hobby farms often raise multiple species that can include pigs and poultry, albeit these species may be housed separately.
- The practice of ‘all-in all-out’ production is not universal and in farms with continuous grower stocking systems influenza transmission is easily supported. Reassortant events among SIV may be enhanced in farms that receive young grower pigs from different sources. Elimination of SIV in farrow-to-finish operations is extremely difficult as there is a continuous supply of a naïve population as new piglets are born into the herd.
- Health status of incoming pigs is a big contributing factor in influenza introductions.
- In Canada, there are far fewer agricultural fairs in which pigs are present compared to the USA where agricultural 4-H and State Fairs are very popular. Pigs exhibited at the Royal Winter Fair are behind glass and cannot be touched by the public. In some petting zoos, Vietnamese pot-bellied pigs may be present.
- There are mechanisms in place to prevent sick pigs from going to market as well as operational plans to prevent mixing or hog shipments between production pyramids.
- The CSHB’s National Swine Farm-Level Biosecurity Standard was developed along with a detailed User Guide. Virtually all of Canada’s swine practitioners were trained and over 90% of the industry completed the National Biosecurity Training Program.
“The swine industry is generally zealous about biosecurity.”
“Biosecurity is critical but may not be enough to completely keep a herd free of influenza.”
6. Laboratory detection of unusual influenza in humans and pigs
An unusual influenza virus constitutes a virus that is:
- an unusual strain
- occurs in an unusual population or demographic group
- causes more severe illness than is typical for that strain
An unusual influenza strain that is spreading more readily than expected in the population is a potentially pandemic strain. Zoonotic influenza in humans is detected by public health laboratory isolate typing. There are 2 ways to characterize influenza virus:
a. Antigenic/serologic
Specific human influenza virus exposure is typically identified with the Haemagglutination Inhibition test (HAI) that can also quantify virus titres. The titres are highest with the closest genetically matched virus strain. In swine, a serum ELISA test is more typical.
b. Genetic
The HA (haemagglutinin) and NA (neuraminidase) influenza virus components are genetically sequenced. The sequences are then compared to gene trees to detect a match. Initial RT-PCR uses Flu Panel reagents to detect presence of influenza A or B viruses and to subtype to the HA level. RT-PCR can be done by the next day. Genetic sequencing to determine the exact HA and NA proteins can take several days. Some public health laboratories are able to characterize both human and swine origin influenza strains. If all human laboratories had current animal influenza panel reagents and if animal laboratories had human influenza panel reagents, the detection of a novel influenza might be detected more rapidly. The sensitivity of the test can be enhanced through the use of a more complete panel of reagents. Unusual influenza strains will be recognized as untypable. They will appear on the influenza ‘map’ as different than the usual current strains. Virus isolation can take 3-4 days.
In at least one province, both human and swine influenza virus laboratories store isolates for further characterization if necessary.
“Collectively, we could do a better job of illustrating to animal owners the potential cost to them of all infections shared by animals and people. Such an approach (emphasizing lost production efficiency) may do more to encourage animal owners to tighten up their farm biosecurity and policies preventing sick employees coming to work than trying to convince them of their responsibility to prevent mixing of infections in their workers or animals.”
6.1 In humans
For human cases, a swab sample is sent to the district laboratory to be identified as influenza A or B virus. The A viruses have a surface matrix (M1) protein whereas the B viruses have a different target, the NF1. Influenza A viruses are subtyped to H1 or H3 seasonal flu, or pH1N1. This diagnostic process takes 3-5 days. Positive influenza A samples are entered into the FluWatch sureveillance data. The NML receives and characterizes 10-15% of the influenza A samples to determine if the strains are antigenically similar to the strains contained in the seasonal vaccine. The publically available GenBank can be used as a reference map of influenza viruses. This subsequent genetic sequencing might take up to 3-4 days. Provincial public health laboratories in large provinces (e.g., QC, ON, AB, BC) can sequence the HA portion of the influenza virus. Any significant differences from the vaccine strains and any unusual strains are immediately and automatically sent to the NML for further characterization. Provinces without the capacity to subtype the influenza viruses send samples to the NML directly. The NML determines and communicates the national and regional variation in influenza strains under the FluWatch surveillance program.
6.2 In swine
For swine cases, veterinarians or researchers submit nasal swabs, saliva, or lung samples for virus isolation and/or virus identification. Research projects may also collect saliva on ropes as samples. Initial identification of influenza A is conducted with PCR matrix for type A, with results typically reported within 2 days. The process for identifying an unusual influenza A virus is conducted through HA and NA subtyping using serologic assays and molecular detection and subtyping. A provincial or regional animal laboratory conducts subtyping to H and N level and partial genome sequencing of H (H1,H3), N, M. Virus isolation utilizing cell culture and embryonating SPF chicken eggs can be conducted at the NCFAD reference laboratory and at some other animal health laboratories.

Samples submitted by local veterinarians to any Canadian Animal Health Surveillance Network Laboratory (CAHSN) are submitted to the NCFAD reference laboratory for full genome sequencing (all 8 segments) if an unusual SIV strain is detected. An unusual strain is suspected if infection is associated with novel clinical signs, a novel epidemiologic pattern, or if an outbreak in swine is associated with illness in a human. Such submissions are rare. From time to time, as was done in 2009 or for specific research projects, the NCFAD characterizes SIVs submitted by the network labs. Reporting times vary from one day to three weeks depending on the depth of analysis. NCFAD influenza sequence results are submitted to the GenBank and in that way shared with other laboratories. For some samples originating from western provinces, further characterization of viral strains may be conducted at the University of Minnesota or Wisconsin. Eastern provinces without provincial animal health laboratories submit samples to QC or ON provincial animal health laboratories. NCFAD laboratory results are reported to the submitting laboratory director and to the appropriate persons in CFIA and PHAC if appropriate. The cost of animal influenza testing varies depending on the type of test, the degree of testing, and which laboratory is conducting the analysis.
- PCR $17–38
- Virus isolation $40
- Antigen ELISA $26
- Antibody H1 (H1N1, H3N2) $9
- Antibody H1 (H1N1, H3N2, multiscreen) $9
- Haemagglutinin typing PCR $38–46
- Sequencing $130–200
Typically, veterinarians submit samples for influenza testing on behalf of their producer clients but producers themselves may submit whole carcasses. Producers of breeding stock submit samples to their veterinarian for submission to the lab for testing. Who pays for the SIV testing varies by province and the circumstances under which the testing is conducted. If nasal, saliva, or blood samples are submitted to the laboratory, generally the producer pays for the influenza testing. However, if a whole carcass is submitted to an animal health laboratory operated by the provincial Department of Agriculture, there may be a flat fee (e.g. $100) to the producer for diagnostic pathology that may include SIV testing at the discretion of the pathologist. Producers pay for the full cost of whole carcasses submitted to private laboratories. In Alberta, where SIV is notifiable, initial testing is paid for by the producer; additional testing for genetic characterization is covered by the provincial government. In one province the cost of influenza testing is split between the province and the producer/veterinarian (i.e., public 75%; private 25%) provided the testing is done through the provincial animal health services laboratory. If testing is conducted as part of an outbreak investigation, the entire cost is borne by the province. In another province, testing at the provincial animal health laboratory is subsidized approximately 50% by the government. For a time limited period, during the H1N1 pandemic, all laboratory fees for influenza testing were covered by some provinces. In Quebec, testing of nasal swabs, saliva, or lung samples for influenza submitted to a Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) network laboratories is free under a current year-round testing program. However, this program may be time-limited.
“Free swine influenza testing for producers, providing that it could be offered on an anonymous basis, may help with ‘buy-in’[to influenza surveillance].”
“If producers support the regulatory response to a positive finding then the offer of free testing might help increase submissions.”
Though it is broadly acknowledged that freely available testing could increase the number of samples submitted, it is possible that only the number of samples per herd would increase and not the number of herds tested. Until the depopulation of the Alberta herd, during the H1N1 pandemic, free testing resulted in increased submissions. After that time, some provinces experienced a temporary decline in submissions and some submissions specifically stipulated “only test to Influenza A level, do not type further,” despite the availability of free testing. Quebec, a province with a long standing trusting relationship between the swine industry and the provincial animal surveillance team, experienced an increase in testing during the 2009 influenza pandemic when testing was offered for free. In one province, some feed companies cover laboratory testing costs in order to determine if the feed is the source of a health problem. Under these conditions more influenza testing is conducted. Distance of the farm from a laboratory is also a factor in limiting the number of submissions. In the case of fatalities, producers may submit the whole carcass to the laboratory. This is only practical if the producer is located close to a laboratory. Otherwise, samples are taken, typically by a veterinarian and shipped to the laboratory, which can be costly.
A Gap Analysis performed by the CSHB to investigate the effectiveness of Canada’s veterinary laboratory system in detecting emerging swine diseases showed that Canada’s labs are well equipped and able to detect emerging strains relatively quickly. The Gap Analysis revealed that the number of submissions is a limiting factor. The cost of testing was identified as an important factor limiting submissions.
7. Communications and materials sharing among laboratories
The NCFAD laboratory works closely with their NML human pathogen counterpart. Communication usually occurs at a high level (i.e., Lab Director or above). During ongoing investigations (e.g., during the pH1N1 outbreak), communication is much more extensive and at many levels including the lab scientist level. A great deal of sharing of reagents between animal and human laboratories occurred and will likely continue to occur. Communication and sharing of reagents between human and animal health laboratories at the regional level may not occur to the same extent as at the national level. Participant laboratories reported that the regional and provincial animal health and public health laboratories communicate informally as-needed. One provincial animal health laboratory reported that they had provided animal influenza virus isolates to the provincial health lab for test development purposes.
During the pH1N1 outbreak, the NML/ NFAD laboratories, CFIA, and PHAC were instrumental in developing the capacity of some provincial, regional, and university research animal laboratories for PCR testing of pH1N1 in animals. At the laboratory level, the biggest challenge for making the epidemiological link between a case or outbreak of swine influenza and a case of variant influenza in a person is confidentiality, which makes information sharing difficult. More coordination between departments of public health and departments of agriculture could be helpful. While one respondent suggested federal coordination and maintenan ce of a swine influenza database, another pointed out that surveillance and response is best handled at the provincial level. It was recognized that information sharing between the NFAD (CFIA) laboratory and Ministries of Health could be improved.
8. Surveillance of influenza in humans and in pigs
Laboratory surveillance must be conducted in specifically designated separate human or animal laboratories. Whole influenza viruses cannot be shared between human and animal laboratories due to strict regulations; however, noninfectious viral components can be shared. Because resources are limited, it is vitally important to be clear about the goals of any surveillance. Two key questions are: “Why are we doing this?” and “Why are we doing it this way?”
Real challenges exist in dealing with surveillance of zoonotic diseases partly due to difficulty with data sharing resulting from privacy legislation. Nevertheless, confidentiality needs to be respected by all parties. Though statutory requirements dictate confidentiality, these can be different for public health investigations versus academic research. One researcher suggested that viral genetic sequencing surveillance for purposes of research could wait until the end of the study so as to help protect confidentiality. Influenza research at the human-swine interface should separate surveillance for research from the case management of an unusual influenza strain. In the case of SIVs in swine, reporting is dictated by provincial regulations.
An integrated human and animal influenza surveillance program, in which more samples will be sequenced, is being planned for Quebec.
Though it is impossible to predict the occurrence of a novel strain that can lead to a pandemic, comparing the swine and human strains as they evolve may be helpful. It is believed that the pH1N1 was circulating among pigs for a few months before it crossed the species barrier. A better understanding of virulence and transmission factors could help.
“The more we understand about these viruses the better. The scientific knowledge could be beneficial.”
“When you look; you will find.”
8.1 Influenza surveillance in humans
• The ultimate goal of surveillance of any disease in humans is to reduce morbidity and mortality.
• Influenza is tracked through regular channels of surveillance and through periodic research projects. During the H1N1 pandemic there were a number of research projects funded through PHAC and CIHR. One project involved sampling any hospitalized patients exhibiting heart or lung disease; reports were shared with federal and provincial jurisdictions.
Unusual Influenza in humans is reportable to public health authorities in Canada. Since the H1N1 pandemic, surveillance has been enhanced through the FluWatch program in Canada (http://www.phac-aspc.gc.ca/fluwatch/) and a similar program in the USA. The ability to detect novel strains more quickly has improved. Increasing availability of rapid gene sequencing has also helped. Communication among laboratories and between laboratories and public health has also improved.
FluWatch consists of a network of labs, hospitals, doctor’s offices, and provincial and territorial ministries with the goal of providing timely current information on flu activity across the country and monitoring circulating strains. It includes:
• Tracking of hospital discharge diagnostic codes.
• Tracking of sentinel physician networks in which a designated physician at various sites across Canada records the number of ILI cases meeting a case definition and samples a subset of this population by sending swabs to the Provincial Public Health Laboratory. Once there, the samples undergo basic PCR testing to determine the presence and type of influenza virus.
Flu Watch has 2 arms:
Diagnostic arm:
- Family doctors take swabs from people with ILI.
- Early and in the middle of the flu season these samples are cultured and characterized (HA sequenced) more intensively to determine if the seasonal flu vaccine strains match the circulating strains. Influenza virus isolates are also monitored for changes throughout the season.
Surveillance arm:
- One day a week, participating family doctors track cases of ILI as a proportion of all cases that day.
- A proportion of swabs taken of people with ILI are characterized at the NML.
- On a monthly basis the NML issues a report on the proportions of circulating subtypes, their lineages, and their genetic match to vaccine strains.
- Laboratories evaluate the distribution of flu viruses by type and subtype compared to other respiratory viruses.
Under routine surveillance, information about animal contact and travel history may not be collected unless an investigation is triggered by the detection of an unusual strain. The usual information accompanying a laboratory sample is age, sex, and patient postal code.
In the event of the identification of an unusual strain, the laboratory will report the finding to the CMOH, who would inform PHAC. The CMOH in the province where the patient resides conducts an investigation. A bulletin is sent to physicians in the community asking them to be vigilant and to be sure to include information about patient animal contact and travel history along with their sample submissions. For example, physicians were notified within a few days of the case of an Ontario man who became infected with variant H1N1 following contact with pigs, Sept. 2012.
Through the FluWatch program Health Canada produces weekly reports about cases over the previous 2 week period. Flu Watch also reports weekly on laboratory detections through the Sentinel Laboratory Respiratory Virus Detections Surveillance System (RVDSS).
8.2 Influenza surveillance in swine
Producers are interested in any knowledge that can help them improve their biosecurity and welcome constructive feedback. A deeper understanding of how influenza virus is spread could help them with biosecurity.
In the event of a case of zoonotic influenza, the response, and associated economic impact of influenza surveillance for public health purposes, needs to be carefully considered, especially because the response can impact the swine industry’s willingness to co-operate. The industry concerns include anonymity and clarity about a response. The industry alone should not bear the cost of SIV surveillance for public health purposes.
• The utility of SIV surveillance for PH purposes is limited but it would help identify specific groups of swine workers who should be vaccinated.
• SIV surveillance might be useful to determine spread of a virus through specific production chains or between barns in different production chains.
• Sampling would be triggered by the herd veterinarian noticing severe ILI symptoms in specific herds or production chains or through laboratory detection of a novel virus in the human surveillance system.
• Sampling protocols would have to be in place prior to any outbreak.
• Sampling would need to be done in a timely manner as the window for detection is very narrow.
• Any response should be targeted and focused on production facilities downstream from the initial detection or index barn.
• Confidentiality concerns for the workers, other people directly involved, and the swine industry itself would need to be addressed.
“Care must be taken in any surveillance strategy to avoid the appearance that swine and pork production are to blame for the emergence of pandemic influenza. There is not enough evidence to support this view, but some public health documents leave this impression. This perception could put an industry out of business.”
There is broad recognition that it is important to understand the evolution of SIV through gene sequencing. Flu strains in pigs, classic H1N1, H3N2, and H1N2 strains, have been relatively stable though new reassortants are expected to emerge. Some strains have had a greater health impact than others. The pH1N1 appears to cause fairly mild disease in pigs. In the USA, a recent variant H1N2 (H1N2v) [an H1N2 reassortant that contains the M gene from pH1N1] infected 3 people in Minnesota USA. http://www.cdc.gov/flu/spotlights/h1n2vcases-mn.htm In swine, this new H1N2v appeared to cause more severe disease and last longer in individual animals and within herds than some of the swine H1N2 (swine H3N2 with pH1N1) strains.
“If the evolution of SIV is monitored closely and reported upon regularly, then it may not be considered as news but rather just part of regular disease monitoring of a sensitive and robust surveillance system.”
“We need to overcome stigma attached to surveillance and make it a benefit to the animal health community/producer and the public health communities. There would need to be coordinated efforts between public health and producer groups.”
Surveillance of SIV among swine is seen by the industry from two viewpoints. On the one hand being more transparent than your competition may be seen as being naive but on the other hand, it is better to be fully transparent for export markets. If there is a lack of confidence in our transparency and ability to respond it can take longer for trading partners to believe federal Canadian authorities regarding a disease status. The Canadian swine industry relies heavily on the export market. The industry accepts that it is important to be vigilant and transparent.
At the same time, not all producers rely on export markets and no producer wants to be blamed for introducing a new pathogen. Rumors or news of influenza in a herd can lead to immediate loss of buyer contracts at the processing plants. For these reasons some producers may prefer not test for influenza at all. Uneven testing across the country of swine diseases can result in unfairness. For example, if one province is testing for and finds a particular disease, then the breeding stock can be shut-out of other provinces that do not test and report on that disease.
Four key aspects of surveillance need to be considered:
• Confidentiality/anonymity
There will be more industry cooperation if information identifying specific farms is not collected or shared, providing there is no danger to animal or human health that would require some restrictions on the farm. It would be best to address any surveillance of the evolution of SIV on a large regional scale or national level rather than at the individual farm level.
• Data quality
It would be best to monitor influenza (including occurrence of new strains and prevalence of ILI) through preexisting surveillance (at least on the animal side), since the value of a separate targeted SIV surveillance system is extremely low.
• Communication
Clearly defined scope of the communication and recipients. Communication should be regular and timely.
• Response Government/public health: producer and veterinarian suspicions of regulatory over reaction, as perceived in 2009 with the depopulation of the Alberta pH1N1 swine herd, significantly damaged surveillance monitoring of influenza types in swine, albeit this depopulation was done at the request of the producer (Howden et al., 2009).
“It can take time to rebuild goodwill and trust.”
“Any surveillance and prevention measures regarding novel zoonotic pandemic influenza viruses would require industry and swine worker ‘buy-in’.”
The principal reasons for SIV testing:
(a) Clinical cases.
(b) Regular herd monitoring.
(c) Determination of health status for export/sale (i.e., to prove absence of disease).
“The full confidence of our trading partners depends on Canada’s transparency regarding animal health.”
In Canada, non-H5/H7 influenza in non-humans is not reportable to the CVO with some exceptions. In Quebec and Alberta, swine influenza A is notifiable by laboratories, reportable in British Columbia. In Ontario, starting in 2013, all swine influenza A is an Immediate Notifiable Hazard. In these latter three provinces under Animal Health legislation, SIV reporting is not anonymous (i.e., includes producer/farm identification). Under the regulations, virus subtype does not need to be reported. In Alberta, both suspect and confirmed cases must be reported to the CVO by the producer or their herd veterinarian. In Quebec, effective surveillance of farm animal diseases is accomplished through a network of three government animal health laboratories, a university animal health laboratory, and a private laboratory that together conduct a high proportion of all animal testing in the province.
In provinces in which SIV is not reported to the CVO, cases are not required to be reported unless they appear to:
(a) Cause significant increased illness or severity of clinical signs in the affected pigs.
(b) Are linked to ILI in swine workers.
(c) Are identified as novel strains in laboratory tests.
Monitoring SIV antibodies is conducted for health monitoring and to establish vaccine status. It may also be done for specific research projects. In these cases, blood samples are typically sent to private or research animal laboratories.
Challenges to SIV surveillance:
• Producers and veterinarians may fail to report cases for several reasons:
- Fear of herd quarantine after the H1N1 2009 case experience
- Inconvenience of completing and submitting the required forms
- Influenza symptoms are often mild and self-limiting, thus can be missed
- Influenza symptoms can be similar to a number of other respiratory diseases (including poor air quality).
• Effective use of resources for surveillance would be best served by having a comprehensive system that can be altered to produce information for diseases such as influenza. Once a surveillance system is operational, roughly the same amount of effort to collect and report on one disease can be applied to many.
• Producers wish to know clearly what the response will be should a zoonotic influenza virus be detected in their herd.
• In a user pay environment, the number of laboratory submissions represents the degree of uncertainty about a disease problem. Typically, testing is done only when:
- There is a new problem (e.g., the veterinarian suspects influenza but the presentation is atypical (e.g., chronic ongoing poor performance).
- There is difficulty controlling the problem (e.g., chronic problem in nursery pigs or mixed infections with other pathogens) and a diagnosis required in order to recommend vaccination or other management changes.
In a typical acute outbreak that resolves rapidly, samples are often not taken.
• In general, laboratory submissions decline once the veterinarian is confident with the diagnosis and control measures are implemented. A further reduction in submissions occurs when the producer recognizes the problem and implements control measures without the involvement of the veterinarian.
• Veterinarians may make diagnosis on clinical signs alone or submit some samples to verify an influenza diagnosis. Often producers are not willing to pay for or proceed with more detailed subtyping (i.e., just test to Influenza A level).
• Passive surveillance cannot represent the true prevalence of cases or detect early trends.
- Breeder stock producers may wish to demonstrate freedom of infection
- Breeder stock producers, in the event of an outbreak, are more likely to submit samples than producers of grower pigs in which the consequences of SIV are less consequential.
- There are relatively fewer breeding stock producers than grower farms.
• Only specifically designed research studies can reveal the true prevalence of SIV in the Canadian herd.
• In general, on a routine basis subtyping with PCR and sequencing if necessary is conducted only at the submitter request, ususally only if the clinical history is unusually serious (eg. higher mortality in animals).
• One province pays for the characterization of a random subset of submissions, with permission of the submitter, and has the authority to characterize any unusually serious cases, and cases with a known link to a human case.
A World Organization for Animal Health (OIE) requirement of countries that trade in live animals and their products is effective animal disease surveillance. Rapid identification of emerging or introduced pathogens is the basis for a rapid response to pathogens of economic and/or public health consequences. In 2002, the CFIA established the CAHSN to facilitate exchange of information about animal health diagnostic trends, techniques, and research, to identify common issues of concern, and to improve linkages among organizations and scientific staff involved in animal health diagnostic work in Canada. CAHSN, a network of federal, provincial, and university animal diagnostic laboratories has improved the national capacity to detect emerging animal diseases in real time, including introduced foreign animal disease. All participating laboratories meet national standards of quality assurance and biocontainment. The ‘Smart Engine’ information technology developed for PHAC’s CNPHI web-platform was adapted to CAHSN facilitating data collection from disparate laboratory systems for analysis by those with access to the CAHSN web-based platform. For laboratories not yet linked to this standardized system, in the event of an outbreak, CAHSN has the flexibility to readily and rapidly establish an interim linkage (Kloeze et al., 2010, Kloeze et al., 2013). The CAHSN primary working group includes the Canadian Animal Health Laboratories Network, Council of the Chief Veterinary Officers, and the National Influenza Collaboration for Animal Health. The National Centre for Foreign Animal Disease (NCFAD) in Winnipeg, Manitoba acts as the network center.
“The purpose of CSHIN is to identify change.” “It is a bottom up network that has proven sensitive in detecting new flu strains which cause more severe disease in pigs than previous strains.”
As a national animal health network, CAHSN focuses on animal disease threats with zoonotic potential and can provide a rapid response to minimize human health and economic risks to Canada. CAHSN has direct links to public health agencies though CNPHI facilitating nation-wide simultaneous alerts to public health and animal health professionals. Through CNPHI, provinces can monitor SIV laboratory data submissions. Significant findings, such as multiple strains in a clinical case, or the occurrence of a novel strain triggers closer monitoring (http://www.inspection.gc.ca/english/agen/broch/sciwortrav4e.shtml#tc42a). The infrastructure and partnerships established by CAHSN facilitated rapid identification of pH1N1 in an Alberta swine herd and led to the notification of OIE in May 2009. CAHSN was able to begin monitoring SIV test results from across Canada within 3 weeks of the WHO announcement of this new influenza strain. CAHSN laboratories reported a sharp increase in submissions for SIV testing in the month of May 2009. CAHSN is also supported by a formal network of epidemiologists and disease control experts from various jurisdictions across Canada through the Surveillance/Epidemiology Advisory Committee (SEAC) created under Canada’s Council of Chief Veterinary Officers. A more formal data sharing agreement is being developed within CAHSN to address cross-jurisdictional reporting.
In Quebec since 2003, in addition to laboratory surveillance of swine diseases, an expert network of 10 swine health professionals with varied expertise including microbiologists, pathologists, epidemiologists, swine veterinary practitioners, and academics meet quarterly via conference call to discuss trends in swine health. Preceding these meetings all provincial swine veterinary practitioners are invited to complete a structured survey to document their clinical impressions regarding specific swine diseases including SIV. In this way the expert network can identify and monitor clinical impressions about swine disease frequencies and trends. Following each expert network meeting, a report is sent to all provincial swine practitioners, outlining current trends based on the experts’ evidence and laboratory submissions, recommended control measures are discussed. The benefit to swine practitioners from this timely information has helped to build trust and confidence among the veterinary practitioners, the expert network, and MAPAQ surveillance teams. This has led practitioners to routinely make regular timely formal submissions of information and punctual informal reporting of any unusual disease occurrences that may require a full investigation by the CVO and MAPAQ surveillance team.
The expert swine health network in Quebec informed the model, in part, for a new national program, the CSHIN, created by the CSHB to collect information on disease trends in swine and to make recommendations for further action.
The network conducts two complementary activities:
a. A social network of swine health clinicians submits their impression of any changes in disease trends that they are witnessing. Impressions are based on syndromic information including coughing, mortality, etc. and which production groups are affected (e.g., sows, boars, gilts, weaners, growers, finishers) and veterinarian assessments (differential diagnoses). Syndromic information is collected through farm visits and any other communications with producers. This activity is currently paid for by CSHB.
b. The collection of laboratory data submitted by veterinarians to the network. These data are collected passively from submissions made by veterinarians and paid for by producers. Laboratory submissions represent a fraction of the actual disease prevalence or farm visits by veterinarians.
“The producer gains because their veterinarian is better equipped.”
CSHIN includes approximately 17 participating swine veterinary practices across Canada, comprising 50% of the national herd and representing three regions: Western, Ontario/Maritimes, and Quebec. A quarterly report by region is circulated to the participating veterinarians. The release of the quarterly report is followed by a regional-level teleconference among participating veterinarians and experts to identify and review new health problems as discussed in the quarterly report. In a subsequent national teleconference with representatives from each region, regional and national trends are identified. In consultation with the expert network, industry groups, and representatives from government agencies, recommendations for action are discussed. A short report with highlights from the national quarterly meeting and practical advice to practitioners is widely circulated among all swine veterinarians and producer organizations. In addition, from time-to-time CSHIN has produced or commissioned disease reviews to help veterinarians and producers stay up to date with new information regarding diagnostics and control measures.
Anonymity of data is crucial to producers, though participating veterinarians and laboratories know the farm identity, data submissions to CSHIN are anonymous unless the producer permits the veterinarian to disclose premise identity or the disease is provincially reportable or notifiable. Because CSHIN is built from the bottom up with minimal government involvement, producers feel like they have some control of the data. The long term goal for CSHIN is to link the expert impression data with laboratory data directly, if possible. Timeliness of data submissions is also crucial to CSHIN’s success. Timely submissions of data approximate near real time data coming into the system. Currently, contracts with participating veterinarians require web-based submissions regarding practitioner clinical impressions based on farm visits, phone or e-mail consultations with producer clients, and laboratory results within 7 days. In cases of influenza, veterinarians may request that the producer submit samples directly to the laboratory. These samples may include ropes (ropes placed in the pens are chewed by the pigs, in this way saliva samples can be collected). In the future, data submissions will be required within 72 hours to be eligible for full compensation to submitting veterinarians. A similar system for cattle practitioners in Alberta requires data submissions within 60 hours.
There are 2 key criteria for veterinarians to make timely data submissions:
• Veterinarians need compensation for time taken to make the submissions.
• Veterinarians need to receive value from the information submitted, value they could pass onto their clients through better practice.
If the information gained through the surveillance system is not timely, not shared, or not presented in a useful way then value is diminished. Thus far, the response from most participating veterinarians has been positive. In the initial stages of the program there has been some reluctance in sharing data on the part of the veterinarians so results may not include as much detail as may be known to them. For example, though the laboratory results may indicate pH1N1, only ‘influenza’ is declared as the laboratory result. It is anticipated that as trust builds over time more detailed information will be shared. The experience with the swine expert network in Quebec has demonstrated that it took time to build the network and integrate it successfully into veterinarian regular practice tools. However, once veterinarians relied on the network they became more proactive about submitting information making the network more sensitive in detecting problems. CSHIN aims to have a similarly strong relationship with its participating veterinarians. Data sharing is enhanced if industry takes ownership of the surveillance rather the government. CSHIN aims to rival Denmark’s reputation for a trustworthy and sensitive system of swine disease detection and control. The success of the CSHIN program depends on its perceived value by participating veterinarians and their producer clients. Knowledge transfer through the quarterly reports, periodic studies, and by veterinarian participation keeps all veterinarians and producers up to date on the latest trends, allowing veterinarians to make better decisions and utilize laboratory testing more effectively.
The CSHB is building governance structures for CSHIN that will include, on an as-needed basis for decision making, government representation from: provincial CVO offices, Agriculture and Agri-Food Canada, and the CFIA. Government involvement will also serve to keep government directly informed about what is essentially a bottom-up network. CSHIN surveillance will form the basis for a coordinated response to new problems that may include a position statement, public communications, a call for specific research projects or investigations, a call for tighter and specific biosecurity measures, or a specific emergency response. As government agencies recognize the value of the network they are likely to become more involved.
Funding for CSHIN was guaranteed up to November 30, 2013. The federal government indicated their support for this model of industry initiated surveillance structures. Funding models for sustaining the project is currently being explored and may include funding from some or all stakeholders: swine industry stakeholder groups such as the Canadian Association of Swine Veterinarians, Canadian Meat Council, Canadian Centre for Swine Improvement Inc., Canadian Swine Breeders Association, PigGEN Canada, Canada Pork International, Canadian Pork Council, Canadian Swine Exporters Association, and government agencies.
9. Communications among government agencies, laboratories, and industry groups
All provinces reported good working relationships between their Ministry of Agriculture and swine veterinarians that would lead to informal information sharing regarding unusual influenza strains regardless if swine influenza were reportable or notifiable. In the event of an unusual strain, this informal information sharing may extend to the CMOH.
Provinces reported good lines of communication between the Ministries of Health and Ministries of Agriculture. The provincial CVO or delegate and the CMOH from each F/P/T hold joint face-to-face national annual meetings to discuss common issues. Generally, the CVO and CMOH understand that there can be an overreaction to SIV. Unusual laboratory results are communicated to the CMOH and CVO from the provincial public health lab and animal health labs respectively. The CMOH and CVO communicate directly by phone or email on an as needed basis. For example, in one province non-H5/H7 influenza A found in animals doesn’t need to be reported to the CMOH but as a courtesy the CVO does report the case without producer identifying information. Though there is no legal requirement for the CMOH to inform the CVO about an ILI or variant SIV in a human, in practice the good working relationships between the provincial CMOHs and the CVOs lead to such communications.
In one province, the Ministry of Agriculture and the Ministry of Health surveillance department both have a public health veterinarian position. In addition there are joint projects between the two agencies leading to strengthened professional and personal relationships.In one province there is an annual zoonotic disease symposium attended by public health and animal health professionals alike. In another province, the Ministry of Health and Ministry of Agriculture improved informal working relationships and lines of communication between the two ministries through One Health joint projects that include sharing of confidentially protected data and analytical capacity. Another province has a cross jurisdictional Zoonotic Disease Committee that meets 2-3 times a year. In some provinces, public health authorities consult with Zoonotic Disease consultants and Ministry of Agriculture surveillance veterinarians. In Quebec, a formal Zoonotic Diseases Agreement between the Public Health and Animal Health Authorities has been in place for many years. Under this agreement exchange of information, including personal identification information is permitted in cases of laboratory identified zoonotic pathogens. In the event of a human case of zoonotic disease linked to domestic livestock, the information sharing triggers a MAPAQ investigation in which MAPAQ works with the producer to mitigate the disease in animals and prevent 36 further transmission to humans. Similarly, cases of an animal infection with a zoonotic agent results in information sharing with the public health authority and a subsequent investigation or enhanced surveillance as needed. In cases of potentially zoonotic influenza, all provinces exchange information commensurate with the perceived threat.
Representatives from the Canadian Animal Health Surveillance Network (CAHSN), comprised of veterinary laboratories across the country, meet regularly. Provincial and CFIA representatives may attend these meetings.
The National Farmed Animal Health and Welfare Council, a partnership among government, industry, and NGO groups meets every few months by teleconference and periodically in face-to-face meetings.
The governance structure of CSHB provides for representation of swine industry sectors, plus Agriculture and Agri-Food Canada, CCVO, and CFIA. This multi-stakeholder approach provides a framework for effective communication and collaboration on health issues within the sector, and with other animal and human health organizations and initiatives.
Despite a broad consensus regarding good lines of communication between the CMOH and CVO offices, a number of respondents suggested that there was a need for improved communication at other levels. Suggestions included improved channels of communication between CFIA laboratories and public health agencies, and establishing formal communication channels between animal health and public health laboratories.
10. Government response to detection of an unusual strain
In cases of zoonotic diseases, it is important that public health agencies understand the impact of their responses and actions on the swine industry and that the industry understand the constraints faced by public health. Very clear and consistent public messaging is required. Although health care is a provincial jurisdiction, PHAC may need to request information from the provinces. Constitutional and legal issues need to be clearly understood. The identity of farms and animal owners that submit samples needs to be protected. At the same time, given that transmission of influenza between people and pigs and the emergence of reassortant strains is uncommon, measures to prevent transmission should not be onerous and disproportionate to the risk. Otherwise, the measures will not be adequately followed.
“If the virus was easily transmissible between people, then quarantining the swine herd would be of little value.”
The following considerations were shared by all provinces:
1. IF an influenza strain emerged that was as highly pathogenic to swine then it would be a very serious threat to swine health and export markets, regardless if it could spread to humans.
2. IF a zoonotic strain emerged in animals (swine, poultry, or other) that did not cause serious clinical disease or increased mortality in animals and was transmissible to humans but was not worse than seasonal flu in pathogenicity in humans and spread through person-to-person contact then it should be “business as usual” and not cause significant harm to the animal industry. BUT if the perception is that animals are at fault and especially if regulatory officials (public health or animal health) react disproportionately to the threat (i.e., impose unjustifiably severe controls on animals) THEN it could cause severe economic harm to industry.
An aggressive response for animals is appropriate if it causes severe disease in animals (i.e., HPAI in poultry or similarly highly pathogenic influenza in swine or horses). IF the viral clinical impact is severe in animals (i.e., analogous to HPAI), international trade will be impacted and CFIA should lead the animal health response, otherwise provincial ministries of agriculture will lead.
An aggressive response for people is appropriate if a zoonotic novel influenza strain causes serious illness in people and is easily transmitted among people. But it is of little value to pursue control of the virus in animals to protect people. It would be better to focus on general biosecurity, PPE of animal workers, and vaccination of the general public and animal workers to the new strain.
IF a novel zoonotic influenza strain has already been detected in animals elsewhere in the world and we understand how it behaves in animals and humans then the respective Ministries of Agriculture and Public Health agencies can lead an appropriate response in proportion to its pathogenicity in animals and people respectively. IF we are unlucky enough to be the first jurisdiction to detect a serious novel zoonotic influenza virus in animals, then a more precautionary approach may be warranted to gain control while data are being collected to learn of its impact on animal health. In reality, sometimes public pressure forces a more aggressive response than is justified and is not effective for improved public health “An important point to make is that production and marketing of swine not be overly affected by Health or Agriculture interventions, particularly for influenzas that have limited ability for human-to-human or community transmission, or where intervention in swine herds would have a limited impact on community transmission of the disease (e.g., pH1N1).” 38 outcomes. The response would range from nothing, to monitoring, to animal depopulation if warranted depending on viral pathogenicity, transmissibility, and ability to cross the species barrier. A full response is likely to involve the CFIA and PHAC as well as the provincial CVO and CMOH offices. Public health and animal health officials must work together in a coordinated way with consistent messaging resulting in a common communication strategy, messaging, and simultaneous updates.
“It is important to the industry that there is clarification as to what would be the actions taken.”
Given the wide range of possible pathogenicity and transmissibility of a novel influenza virus, and that all provinces indicated that the response would be commensurate with the risk to people and/or animals, it would be challenging to communicate clearly to producers the specific consequences of an occurrence of a novel strain detected on a particular Canadian farm.
“Quarantine and loss of income are powerful incentives to not report suspect influenza and not to participate in surveillance.”
In practice, in all provinces, in the event of a novel influenza virus in swine, including provinces in which non-H5/H7 strains of influenza A occurring in animals is reported to the CVO, in general there will be no regulatory consequences to the animal owners, as the main objective is to monitor trends. Herd veterinarians are capable of conducting investigations and are very likely to ask about ILI among human contacts with the herd and recommend that any ill persons follow-up with their doctors. If an alarming trend is observed (e.g., unusually high mortality in animals) the response would be commensurate with the impact of the strain on animal health. The CVO would also help in any way possible with assessment of the risk and offer support to the herd veterinarian and producer in managing any risk. There could be a CVO initiated on-farm investigation if circumstances warrant. The CVO or delegate would have a conversation with the producer directly or through the herd veterinarian about the threat to human health and measures to protect staff from cross-species transmission. Quarantine of the herd, under the regulation, would be highly unlikely.
In cases of variant influenza strain in a human, the CMOH would request the CVO assistance in the investigation which may include enhanced surveillance (including testing) of humans and swine with human contact tracing and information gathering on swine movements, voluntary restrictions in swine movement between barns within operations and enhanced biosecurity, infection control and use of PPE. The CMOH would also immediately notify representatives on the investigation team from CFIA, PHAC, Health Regions, OH&S and industry. Public Health Authorities would investigate the number and severity of any ILI in people associated with the herd(s), plus any increase in ILI regionally where the herd is located. In one province there is public health legislation related to public health hazards that would allow the public health authorities to take certain actions that could include herd quarantine. In the event of an unusual reassortant influenza strain, the consequences of a positive test must be clearly communicated to producers before samples are taken (i.e., will there be a quarantine and disruption in marketing of pigs). There must be compensation programs considered in advance and implemented quickly to reimburse producers of their losses. If the virus is causing severe disease and/or death in people or swine, a case could be made that a herd quarantine order be placed and possibly depopulated. Applying a herd quarantine order until the virus clears requires a clear plan in place ahead of time of how to remove the quarantine.
11. Suggestions for improved prevention, detection, and mitigation of novel zoonotic influenza
A public health response that quickly reaffirms the continued safety of meat products for consumers is always best for the industry. The federal government could perhaps play a role in ensuring that there is a rapid coordinated public health and animal health response to a sporadic case of human variant SIV or to an emergent zoonotic reassortant potentially pandemic strain found in humans. It is extremely important that messages from health regions, the health ministry and other groups are consistent and done simultaneously.
The Ministries of Agriculture believe any actions or programs undertaken for public health purposes should not risk alienating the industry or make it difficult to engage cooperatively in the future. Animal health should always be at the table during any decision making that affects the industry. In summary:
- We should accept that novel influenza viruses will occur with periodic cross species transmissions. We should encourage balanced practices that reduce the risk.
- We should broaden our vision to multiple species and multiple pathogens not just swine and people and influenza.
- We should strive for excellent biosecurity (infection control) on all farms, all the time, to reduce financial impact of endemic animal diseases, threat of foreign animal diseases and zoonotic diseases.
Specific suggestions of some respondents:
“Certainly, farms that have a reported link to a human influenza infection would be investigated by the CVO.”
11.1 Prevention
11.1.1 Flu vaccination
- Swine workers (producers and their families, swine veterinarians, swine workers and their families) should be on the list of flu vaccination priority groups as are poultry workers. As seen in the poultry industry, providing free vaccination has built good will with the industry as public health is seen to be pro-active and helping to protect their health. Providing free seasonal vaccine to swine workers could be good way to build trust and a positive relationship between the swine industry and public health after experiences in the past in which the PH reaction was seen to be excessive.
- Vaccine uptake among swine workers and their families is very low, in spite of company incentives or free vaccines. Efforts need to be made to increase the compliance among workers, especially immigrant workers where language or other cultural barriers may be a problem. Increase on site (or local) vaccinations of swine workers. Acceptance is higher when this occurs. Public health and producer groups should cooperate in flu vaccination programs.
- Vaccination of pigs with regular vaccine strain updates should be implemented and supported by government agencies (i.e., provincial Departments of Agriculture, CFIA, and PHAC, etc.)
- Increased resources available to industry to help off-set costs such as vaccination of swine.
11.1.2 Biosecurity
- On-farm screening of visitors to make sure they do not have ILI upon entry into the barn. Avoid unnecessary visitors with prior direct contact with pigs, particularly international travelers.
- Enhanced knowledge among public health professionals about zoonotic influenza leading to health care professionals advising persons with influenza to avoid contact with animals.
- Enhanced understanding of human cases of variant swine influenza among Ministries of Agriculture, Environment, Labour, CFIA, and the swine industry leading to resources for the industry to improve infection control practices to prevent spread of pathogens within barns, improve recordkeeping, and the development of BMPs to prevent pathogens from being brought in from outside sources (i.e., fomites, aerosolized, bird and rodent transmission, other non-worker sources like contractors, truckers, etc.).
- Public health agencies hosting industry veterinarians at their meetings regarding zoonotic diseases and likewise public health representatives attending meetings with CSHIN. Producer resistance to participate in government run programs may be overcome through a closer working relationship between government and producer groups established through CSHB, including CSHIN, activities.
11.2 Surveillance
- There appears to be precedence in PH legislation with respect to confidentiality, for example, HIV in humans, a reportable disease can be anonymous [or confidential]. These issues need to be reconciled with the requirement for reporting of SIV to the CVO.
- A case could be made for including a question about contact with animals on the laboratory requisition form. This could result in more timely detection of any variant influenza strains or other zoonotic diseases. However, changing questionnaires cost money and takes time. Public Health patient questionnaires fall under provincial jurisdiction.
- Annual or bi-annual surveillance research studies for SIV’s in North America should be initiated that include genome analysis and seroprevalence studies.
- A much faster way to find new strains that cause serious disease in people is to fully work up all hospitalized cases in people, including virus isolation and full sequencing AND ask if they have had direct contact with animals (not just swine, but all animals). Then get animal health officials to follow up on those specifically implicated animals with which the person had close contact and type influenza in those animals.
- Fully work up (isolate and sequence) outbreaks in animals with abnormally serious clinical disease and mortally in animals AND ask if any people in contact with those animals are sick. IF yes, get public health officials to investigate those people.
- We need a single body to take a national lead on collecting samples, isolates, data, and managing the confidentiality issue and communications. Samples could be collected provincially, but the data needs to be coordinated nationally. There needs to be national agreement on consequences to producers for a positive test and how to communicate test results to the industry and to the public. In the USA it is VS under APHIS.
- Bioinformatics and molecular techniques should be paramount for detecting novel viruses.
- Coordination between departments of public health and departments of agriculture (usually responsible for or associated with veterinary labs).
- Results from national surveillance programs with complete genome analysis and seroprevalence in the field will provide information on current and emerging SIV’s with zoonotic and pandemic potential. Researchers would be able to identify trends in the antigenic drifts and shifts within different strains and predict possible new emerging viruses. Along with the field surveillance, update of diagnostic kits (ELISA’s, PCR’s etc) will be required based on the current situation on the field.
11.3 Response
- Improved definition and understanding of roles and responsibilities during an outbreak (i.e., there might be a federal responsibility or role, but if federal level lacks the resources or availability of “horses on the ground”, then the responsibility will fall to the Health Region or provincial Ministry). Ancillary to this is who pays for the response.
- Having a rapid response public health/research team that represents both the human and animal expertise already in place and ready to respond would be helpful. Having the right technical information early on in the decision making process is critical to good decision making. There would need to be funding set aside for the activities required by the team. There would also need to be a pre-approved plan for dealing with confidentiality. There would need to be a plan and funding for dealing with compensation to producers if needed. There is a role for academic researchers in an investigation. Having a current inventory of influenza researchers in both humans and animals plus a searchable database of their research and reports could be very helpful in the event of an outbreak of novel zoonotic disease. NSERC or CIHR could house such information. http://www.cihr-irsc.gc.ca/e/38021.html
- There should be an overall strategy for the public health response to emergent infectious diseases that is well thought out and consistent across the country. What we learned from the SARS outbreak is that the public health human resources became severely stretched. Though a call went out across the country for help, the lack of portability of licensing was a serious impediment. Provincial licensing is a slow process. By planning ahead and respecting reciprocity some problems may be avoided.
References
Canadian Food Inspection Agency. Science at Work. (2011). Responding to Changing Demands Through Integrated Science. Retrieved from: http://epe.lacbac.gc.ca/100/206/301/cfia-acia/2011-09-21/www.inspection.gc.ca/english/agen/broch/sciwortrav4e.shtml#tc42. Accessed on Jan. 10, 2012.
Canadian Swine Health Board. (2013). National Swine Farm-Level Biosecurity Standard: Swine Biosecurity Standard and Biosecurity User Guide. Retrieved from: http://biosecurity.swinehealth.ca.
CDC. Seasonal Influenza. (2012). Information on Swine Influenza/Variant Influenza Viruses. Retrieved from: http://www.cdc.gov/flu/swineflu/. Accessed on Jan. 4, 2013
Gray, G. C., McCarthy, T., Capuano, A. W., Setterquist, S. F., Olsen, C. W., Alavanja, M. et al. (2007). Swine workers and swine influenza virus infections. Emerging infectious diseases, 13(12), 1871.
Howden, K. J., Brockhoff, E. J., Caya, F. D., McLeod, L. J., Lavoie, M., Ing, J. D., et al. (2009). An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. The Canadian Veterinary Journal, 50(11), 1153.
Kloeze, H., Mukhi, S., Kitching, P., Lees, V. W., & Alexandersen, S. (2010). Effective Animal Health Disease Surveillance Using a Network‐Enabled Approach. Transboundary and emerging diseases, 57(6), 414-419.
Kloeze, H., Mukhi, S. N., & Alexandersen, S. (2013). Swine influenza test results from animal health laboratories in Canada. The Canadian Veterinary Journal, 54(5), 501.
Liu, J., Bi, Y., Qin, K., Fu, G., Yang, J., Peng, J., et al. (2009). Emergence of European avian influenza virus-like H1N1 swine influenza A viruses in China. Journal of clinical microbiology, 47(8), 2643-2646.
Myers, K. P., Olsen, C. W., Setterquist, S. F., Capuano, A. W., Donham, K. J., Thacker, E. L., et al. (2006). Are swine workers in the United States at increased risk of infection with zoonotic influenza virus?. Clinical infectious diseases, 42(1), 14-20.
Nelson, M. I., Gramer, M. R., Vincent, A. L., & Holmes, E. C. (2012). Global transmission of influenza viruses from humans to swine. Journal of General Virology, 93(Pt 10), 2195-2203. 46
Poljak, Z., Friendship, R. M., Carman, S., McNab, W. B., & Dewey, C. E. (2008). Investigation of exposure to swine influenza viruses in Ontario (Canada) finisher herds in 2004 and 2005. Preventive veterinary medicine, 83(1), 24-40.
Public Health Agency of Canada. Canadian Communicable Disease Report (CCDR). An Advisory Committee Statement (ACS), National Advisory Committee on Immunization (NACI). (2012). Statement on Seasonal Influenza Vaccine for 2012– 2013, Vol. 38, ACS-2. Retrieved from: http://www.phac-aspc.gc.ca/publicat/ccdrrmtc/12vol38/acs-dcc-2/.
Public Health Agency of Canada. Canadian Communicable Disease Report (CCDR). National Advisory Committee on Immunization (NACI). (2013). Statement on Seasonal Influenza Vaccination for 2013-2014, Vol. 39, ACS-4. Retrieved from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/13vol39/acs-dcc-4/.
Thacker, E., & Janke, B. (2008). Swine influenza virus: zoonotic potential and vaccination strategies for the control of avian and swine influenzas. Journal of Infectious Diseases, 197(Supplement 1), S19-S24.
USDA. Influenza A. Virus. (2008). Related Graphics: Fig. 3. Retrieved from: https://nccid.ca/wp-content/uploads/sites/2/2015/03/SIVfigure1.jpg.
List of respondents
There were 22 respondents in total. No respondents declined to have their names listed but some respondents did not indicate their permission for listing their name, as a consequence these respondents are not listed here.
Swine Health Academics, virologists, and influenza researchers
Dr. Earl Brown – University of Ottawa
Dr. Robert Friendship – University of Guelph
Dr. Daniel Hurnik – University of Prince Edward Island
Dr. Dale Godson – Prairie Diagnostic Services
Dr. Aleksandar Masic – Bioniche Life Sciences Inc.
Dr. Margaret Russell – University of Calgary
Chief Veterinary Officers or their delegates and CFIA representative
Dr. Brian Radke – BC Dr. Gerald Hauer – AB
Dr. Julia Keenliside – AB Dr. David Alves – ON
Dr. Bruce McNab – ON Dr. Betty Althouse – SK
Dr. Kevin Budd – NS Dr. Glen Duizer – MB
Dr. Luc Bergeron – QC Dr. Robert Kerr – NS
Dr. Harold Kloeze – CFIA
Chief Medical Officers of Health or their delegates
Dr. Phil Curry – SK
Industry representatives
Mr. Bob Harding – Canadian Swine Health Board / Conseil canadien de la santé Porcine
Mr. Martin Rice – Canadian Pork Council / Conseil canadien du porc
Questionnaires:
Virologists
The aim of these questions is to clarify the risks, best preventative measures, and control measures regarding a novel emergent zoonotic influenza virus as it pertains to humans and swine.
General questions:
1. Is it correct to assume that a novel zoonotic influenza virus with pandemic potential could arise in a pig co-infected with a human and swine influenza virus if a reassortant event took place?
2. Could a novel zoonotic influenza virus with pandemic potential arise in a human coinfected with a human and swine influenza virus if a reassortant event took place?
3. Is influenza A the only influenza virus that may become a public health problem or are there other influenza viruses that can affect both humans and swine?
4. Is influenza A virus transmitted via air?
5. Knowing that swine influenza viruses (SIV) can infect humans, would a SIV that contains 2009 pH1N1 genetic material pose a greater threat to humans in terms of virulence or ease of transmission compared to previous SIV strains?
6. Are you aware of any current research anywhere in the world on the study of zoonotic influenza in swine or swine workers? (zoonotic influenza defined as influenza viruses from pigs infecting humans and influenza viruses from humans infecting pigs)?
7. What is the current capacity in Canada for research on zoonotic influenza at the human-swine interface?
8. Are pigs and/or humans that are infected with influenza virus infective before they exhibit clinical signs?
Questions regarding prevention, detection, and mitigation of zoonotic influenza:
1. In your opinion, how can swine producers, veterinarians, and the public health community work together to:
a. Prevent the emergence of a novel pandemic zoonotic influenza virus at the human-swine interface?
i. How would you rate the following measures for effectiveness in preventing a novel pandemic influenza virus from emerging at the human-swine interface:
ii. Vaccination of swine workers against seasonal influenza.
iii. Prevention of contact between swine and swine workers that may have seasonal flu like symptoms.
iv. Biosecurity on the farm.
v. Vaccination of swine against SIV.
vi. Other management measures.
b. Detect the emergence of a novel pandemic zoonotic influenza virus at the human-swine interface?
c. Mitigate the impact of any novel zoonotic influenza strains that may be a threat to public health?
2. Can you say what level of confidence you have in these recommendations?
3. Are there other considerations that have been missed in these questions?
4. Are there any other experts that you suggest that I speak to?
5. Are there any publications that you recommend that I review?
6. Would you like to have a copy of the final report when it is complete?
Laboratories that work with influenza viruses of human and/or animal origin
The following questions pertain to the understanding of the laboratory methodologies, challenges, and costs in identifying novel emergent zoonotic and potentially pandemic influenza viruses. In addition, these questions are aimed at identifying the existing collaborations, communication pathways, and opportunities for collaborations between human and animal health laboratories regarding influenza viruses.
In general:
1. What is the process for the identification of unusual influenza A viruses?
2. How much communication occurs between human and animal virology labs regarding zoonotic influenza? 3. How much sharing of reagents may occur between human and animal viral laboratory labs?
More specifically:
1. Do you isolate influenza viruses in your lab?
2. What tests are conducted for virus isolation?
3. Do you test for antibodies to influenza viruses?
4. To what genetic level do you identify the influenza virus in your laboratory?
5. What type of samples do you receive?
6. Where do these samples come from?
7. Who is submits these samples?
8. If your receive samples from humans does any information regarding contact of the patient with swine accompany the sample?
9. If your receive samples from humans is testing for SIV in a human patient routine?
10. Under what conditions would testing for SIV be conducted?
11. How long does it generally take to report results?
12. To whom are these results reported?
13. What are the costs of these tests?
14. Are the results reported to any other laboratories (human or animal)?
15. Are results entered into a database shared by other laboratories?
16. Are there further tests done on the sample(s) to determine the details of the genetic makeup?
17. Under what circumstances would further virus genetic testing be done?
18. What are the costs of doing these additional tests?
19. How long would it take to receive the results of these additional tests?
20. To whom are these results reported?
21. In your opinion, how could the epidemiological link between a case or outbreak of SIV in swine and a case of SIV in a person be made more effectively and efficiently?
22. In your opinion, what are the biggest challenges to making the epidemiological link between a case or outbreak of SIV in swine and a case of SIV in a person?
23. Is your laboratory part of the Canadian Animal Health Surveillance Network (CAHSN)?
24. Is there anything that could be done by other levels of government to help prevent and detect influenza at the swine-human interface?
25. If not already stated, does your laboratory share information or materials with any human laboratories that diagnose influenza viruses?
26. Are there any other considerations that have not been addressed by these questions?
Swine health academics with expertise in swine influenza viruses (SIV)
The aim of the initial questions are to establish the current state of surveillance for zoonotic influenza viruses at the swine-human interface in Canada or elsewhere as well as the current state of research and the capacity for research on zoonotic influenza in Canada.
1. Are you aware of any current surveillance that would detect a zoonotic influenza virus at the swine-human interface?
2. If yes, please describe this surveillance. Is this surveillance on-going or time-limited?
3. Are you aware of any current research anywhere in the world on the study of zoonotic influenza in swine or swine workers? (zoonotic influenza defined as both influenza viruses from pigs infecting humans and influenza viruses from humans infecting pigs)? Can you refer me to any particular research or researchers on this subject?
4. What is the current capacity in Canada for research on zoonotic influenza in swine?
The aim of the following general questions are to clarify the utility of and most effective and efficient methods of surveillance of swine, and swine workers, on the farm and/or at abattoirs and other venues at the human-swine interface for potential zoonotic influenza viruses. The aim of these questions is to also clarify the best methods of interspecies virus transmission prevention and interruption.
General questions:
1. In your opinion, how can swine producers and the public health community work together to prevent, detect, and mitigate any zoonotic influenza strains that may be a threat to public health?
2. Is influenza A the only influenza virus that may become a public health problem or are there other influenza viruses that can affect both humans and swine?
3. In your opinion, would an emergent reassortant swine influenza virus (SIV) that contains 2009 pH1N1 genetic material pose a greater threat to swine workers than previous SIVs? Would it be more likely to be transmitted from person to person than previous SIVs?
Risk factors and prevention measures:
1. What are the most important risk factors for the incidence of SIV in the Canadian swine populations:
• Farm management practices
• Biosecurity practices
• Farm size
• other
2. What are the most effective prevention measures against SIV in swine:
• Biosecurity
• Vaccination against SIVs
• Farm management practices
• other
Can you say what level of confidence you have in these recommendations? Can you refer me to any literature that expands upon or supports your recommendations?
3. What might be the most effective prevention measures against a new pandemic zoonotic influenza strain arising from a Canadian pig farm?
• Vaccination of swine workers against seasonal influenza
• Prevention of contact between swine and swine workers that may have seasonal flu like symptoms (ILI).
• Biosecurity on the farm
• Vaccination of swine against SIVs
• Other management measures
Can you say what level of confidence you have in these recommendations? Can you refer me to any literature that expands upon or supports your recommendations?
Surveillance and detection:
1. Is it important to monitor the evolution of pH1N1 in swine? What would be the best way to do this?
2. Early Detection of an emergent influenza disease (EID):
• Who – should we surveil: swine +/or swine workers
• What – samples, tests, in which animals
• When – regular basis, only if clinical cases
• Where – farm, abattoir, human health care setting
• Would previous vaccination make interpretation of serology difficult
3. Would reporting of incidents of unconfirmed SIV (ILI) among swine be helpful in monitoring SIV infections in pigs?
4. Would reporting of incidents of ILI among swine workers be helpful for monitoring potential zoonotic emergent influenza viruses in people?
5. Do you think that mixed farms that provided product for a small local market belong in a different risk category for emergence of a new zoonotic pandemic influenza virus?
6. Should these farms be treated differently?
Mitigation and response:
1. What would be the best methods to contain an emergent influenza disease (EID)?
2. Who would be responsible for taking action?
Is there anything else that has not been covered by these questions?
Are you familiar with the USDA National Surveillance Plan for Swine Influenza Virus? (The monitoring plan is comprised of laboratory surveillance of pigs exhibiting ILI either on farms or in places of comingling such as auctions or fairs AND surveillance of pigs that are epidemiologically linked to a human with confirmed SIV).
3. Would a similar monitoring plan work in Canada? Please explain.
4. Would a similar monitoring plan work if it was expanded to include swine that are epidemiologically linked to a human with any type of influenza infection, not just SIV?
5. Are there other considerations that have been missed in these questions?
6. Are there any influenza virologists that you suggest that I speak to?
7. Are there any publications that I should review?
8. Are there other people that you know have expertise on this subject that you would suggest that I contact?
9. Would you like to have a copy of the final report when it is complete?
Chief Veterinary Officers and Animal Surveillance experts
(in swine producing provinces: Quebec, Ontario, Manitoba, Saskatchewan, and Alberta, British Columbia, New Brunswick, and Nova Scotia)
The aim of these questions:
1. Is to clarify the role of veterinarians, producers, and provincial regulation in the prevention, detection, and investigation of swine influenza virus (SIV) outbreaks and sporadic SIV, both in swine and in swine workers with particular attention to emergent reassortant zoonotic influenza in swine workers or the public at the swine-human interface.
2. Determine how swine producers, veterinarians, and the public health community can best work together to prevent, detect, and mitigate any novel zoonotic influenza strains that may be a threat to public health.
3. Request your opinion regarding a new reassortant SIV that contains 2009 pH1N1 genetic material: Would this new SIV virus pose a greater threat to swine workers than previous SIVs? Would it be more likely to be transmitted from person to person than previous SIVs? Should all SIVs be fully sequenced to track the evolution of SIVs? Who should take responsibility (i.e., pay) for this laboratory testing?
General questions:
Do you have any general comments regarding how public health and animal health professionals could work together to prevent and detect a novel zoonotic influenza virus from occurring at the swine-human interface in your province?
SIV in swine
Assuming that reportable and notifiable mean different things:
1. Are SIV cases reportable in your province?
a. If so then what immediate actions would be taken?
b. If so then what are the consequences for the producer?
2. Are SIV cases notifiable in your province?
a. If so then what immediate actions would be taken?
b. If so then what are the consequences for the producer?
3. What is the estimated prevalence of SIV cases in your province?
4. Is SIV regarded as a problem to the swine industry in your province?
5. How widely are SIV vaccines used in your province?
6. Under what circumstances are tests for SIV conducted among pigs in your province?
7. Do producers submit samples for SIV testing?
8. Who pays for SIV laboratory testing in your province?
9. If producers pay for SIV testing, in your opinion, would more testing be done if SIV testing were free to producers?
10. Where is the laboratory testing for SIV conducted in your province?
11. Does the province monitor SIVs?
12. Is monitoring the evolution of SIVs done in your province?
13. Is there any monitoring of the pH1N1 virus in swine?
14. Do SIVs undergo molecular characterization (i.e., full sequencing) in your province?
15. If so under what circumstances might this be done?
16. Where would SIV molecular characterization be done for your province? Who would pay for this additional testing?
17. Is there any monitoring of SIV antibodies in swine?
a) If yes, then is this done on a regular basis?
b) If yes, then how is this done?
c) If yes, then where are samples sent?
18. Is there any reporting to the province of unconfirmed cases of influenza-like-illness (ILI) among swine? If so, who would report to the province?
19. Is the province involved in the investigation of a SIV outbreak on a farm or is that done exclusively by the farm veterinarian?
20. Are there any programs in the province to help reduce the incidence of SIV in the provincial herd?
21. If yes, then who sponsors these programs? 22. Is there anything else that needs to be considered?
Influenza-like-illness (ILI) in swine workers
1. Is there any reporting to the provincial agriculture department from human public health laboratories or from public health authorities of confirmed or suspected swine influenza cases among swine workers including swine-veterinarians?
2. In the event of a SIV outbreak investigated by the province, would the investigating government veterinarian ask about ILI among any persons in contact with the swine?
3. In the event of a SIV outbreak investigated by the regular farm veterinarian, would the regular farm veterinarian be likely to ask about ILI among any persons in contact with the swine?
4. Is there anything that the province could do, or is currently doing, to reduce the risk of swine contracting influenza from an infected human?
5. Is there anything the province could do, or is currently doing, to reduce the chance of a swine worker becoming infected with a SIV?
6. If it was demonstrated that immunization of swine workers against the seasonal flu would help prevent a new zoonotic influenza virus emerging from a Canadian herd would the province support a campaign to enhance seasonal flu vaccination among swine workers including swine-veterinarians?
7. If the province already has a program in place to promote seasonal flu vaccination among swine workers, how successful is this program?
8. What are the challenges to increasing seasonal flu vaccination among swine workers?
Emergent novel zoonotic influenza virus
1. Is a novel zoonotic pandemic influenza virus regarded as a potential threat in your province?
2. What would be the impact on the swine industry of a novel zoonotic influenza virus arising from a herd in your province?
3. Would the impact in your province be different if the incident happened in another province or country?
4. In your province under the current practices, how would a novel zoonotic influenza virus become detected?
5. How long might it take for this process?
6. What measures would be taken if a novel pandemic zoonotic influenza virus was epidemiologically linked to a swine herd in your province?
7. Who would be responsible for coordinating these efforts?
8. What would happen to a herd with an epidemiological link to a novel zoonotic influenza virus?
In the United States, the USDA National Surveillance Plan for Swine Influenza Monitoring is comprised of laboratory surveillance of pigs exhibiting ILI either on farms or in places of comingling such as auctions or fairs (pig producers may choose to remain anonymous) AND surveillance of pigs that are epidemiologically linked to a human with Influenza A infection including humans with SIVs or pH1N1.
9. Would a similar monitoring plan work in Canada?
10. Please explain:
Closing questions:
1. Do you think that mixed farms with swine pose a different risk than strictly swine farms for emergence of a novel zoonotic influenza virus?
2. Should these farms be treated differently?
3. Is there anything else that has not been covered by these questions?
4. Are there any other persons that you suggest that I speak to?
Provincial CFIA Animal Surveillance experts
(In swine producing provinces: Quebec, Ontario, Manitoba, Saskatchewan, and Alberta, British Columbia, and New Brunswick.)
The aim of these questions:
1. Is to clarify the role of veterinarians, producers, and provincial regulation in the prevention, detection, and investigation of swine influenza virus (SIV) outbreaks and sporadic SIV, both in swine and in swine workers with particular attention to emergent reassortant zoonotic influenza in swine workers or the public at the swinehuman interface.
2. To determine what are the existing lines of communication within the animal health world regarding SIV in pigs and between the animal health and human health worlds regarding zoonotic influenza viruses and how this communication could be improved.
3. Determine how swine producers, veterinarians, and the public health community can best work together to prevent, detect, and mitigate any novel zoonotic influenza strains that may be a threat to public health.
4. Request your opinion regarding surveillance of influenza in swine that does/would not have a negative impact on the pork industry.
General questions:
1. Do you have any general comments regarding how public health and animal health professionals could work together to prevent and detect a novel zoonotic influenza virus from occurring at the swine-human interface in your province?
2. What considerations need to be included in any policies regarding prevention and detection of zoonotic influenza virus at the swine-human interface?
3. Would surveillance of SIV for evolution of viruses be beneficial to the industry?
4. Would surveillance of SIV for evolution of viruses be beneficial to public health?
5. How could surveillance of swine for influenza viruses be conducted without adversely impacting the swine industry?
6. What role, if any, would/should CFIA have in surveillance of SIV?
SIV in swine
1. Assuming that reportable and notifiable mean different things:
Are SIV cases reportable in your province?
a. If so then what immediate actions would be taken?
b. If so then what are the consequences for the producer?
Are SIV cases notifiable in your province?
c. If so then what immediate actions would be taken?
d. If so then what are the consequences for the producer?
2. What is the estimated prevalence of SIV cases in your province?
3. Is SIV regarded as a problem to the swine industry in your province?
4. How widely are SIV vaccines used in your province?
5. Under what circumstances are tests for SIV conducted among pigs in your province?
6. Do producers submit samples for SIV testing?
7. Who pays for SIV laboratory testing in your province?
8. If producers pay for SIV testing, in your opinion, would more testing be done if SIV testing were free to producers?
9. Where is the laboratory testing for SIV conducted in your province?
10. Does the province monitor SIVs?
11. Is monitoring the evolution of SIVs done in your province?
12. Is there any monitoring of the pH1N1 virus in swine?
13. Do SIVs undergo molecular characterization (i.e., full sequencing) in your province?
14. If so under what circumstances might this be done?
15. Where would SIV molecular characterization be done for your province? Who would pay for this additional testing?
16. Is there any monitoring of SIV antibodies in swine?
a. If yes, then is this done on a regular basis?
b. If yes, then how is this done?
c. If yes, then where are samples sent?
17. Is there any reporting to the province of unusual influenza-like-illness (ILI) among swine? If so, who would report to the province?
18. If the province were to identify an unusual SIV strain in pigs would the human public health officials be alerted? If so how would the communication occur?
19. Is the province involved in the investigation of a SIV outbreak on a farm or is that done exclusively by the farm veterinarian?
20. Are there any programs in the province to help reduce the incidence of SIV in the provincial herd?
21. If yes, then who sponsors these programs?
22. Is there anything else that needs to be considered?
Influenza-like-illness (ILI) in swine workers
1. Is there any reporting to the provincial agriculture department +/or CFIA from human public health laboratories or from public health authorities of confirmed or suspected swine influenza cases among swine workers including swine-veterinarians?
2. In your experience, though this may be a rare event, how long does it take for the provincial agriculture department +/or the CFIA to be notified of a human SIV case.
3. In the event of a SIV outbreak investigated by the province, would the investigating government veterinarian ask about ILI among any persons in contact with the swine?
4. In the event of a SIV outbreak investigated by the regular farm veterinarian, would the regular farm veterinarian be likely to ask about ILI among any persons in contact with the swine?
5. Is there anything that the province could do, or is currently doing, to reduce the risk of swine contracting influenza from an infected human?
6. Is there anything the province could do, or is currently doing, to reduce the chance of a swine worker becoming infected with a SIV?
7. If it was demonstrated that immunization of swine workers against the seasonal flu would help prevent a new zoonotic influenza virus emerging from a Canadian herd would the province support a campaign to enhance seasonal flu vaccination among swine workers including swine-veterinarians?
8. If the province already has a program in place to promote seasonal flu vaccination among swine workers, how successful is this program?
9. Is the seasonal flu vaccine free to all persons in your province?
10. What are the challenges to increasing seasonal flu vaccination among swine workers?
Zoonotic influenza virus
1. If not already mentioned, if a SIV was detected in a human what would be the government response?
2. Is a novel zoonotic pandemic influenza virus regarded as a potential threat in your province?
3. What would be the impact on the swine industry of a novel zoonotic influenza virus arising from a herd in your province?
4. Would the impact in your province be different if the incident happened in another province or country?
5. What measures would be taken if a novel pandemic zoonotic influenza virus was epidemiologically linked to a swine herd in your province?
6. Who would be responsible for coordinating these efforts?
7. What would happen to a herd with an epidemiological link to a novel zoonotic influenza virus? In the United States, the USDA National Surveillance Plan for Swine Influenza Monitoring is comprised of laboratory surveillance of pigs exhibiting ILI either on farms or in places of comingling such as auctions or fairs (pig producers may choose to remain anonymous) AND surveillance of pigs that are epidemiologically linked to a human with Influenza A infection including humans with SIVs or pH1N1.
8. Would a similar monitoring plan work in Canada?
9. Please explain.
Closing questions:
1. Do you think that mixed farms with swine pose a different risk than strictly swine farms for emergence of a novel zoonotic influenza virus?
2. Should these farms be treated differently?
3. Is there anything else that has not been covered by these questions?
4. Are there any other persons or groups that you suggest that I speak to?
Chief Medical Officers of Health
The goals of this survey are:
1. To establish the current state of surveillance for zoonotic influenza viruses at the swine-human interface.
2. To clarify the utility of and most effective and efficient methods of surveillance of swine, and swine workers, on the farm and/or at abattoirs and other venues at the human-swine interface for potential zoonotic influenza viruses.
3. To clarify the best methods of interspecies virus transmission prevention and interruption.
It is hoped that the information provided will form the basis for knowledge, trust, dialogue, and collaboration among the stakeholders involved with a potential zoonotic pandemic influenza virus.
General Questions: Healthy animals – Healthy people
1. In your opinion, how can the swine industry, the public health community, and other government agencies work together to prevent and detect any zoonotic influenza strains that may be a threat to swine health?
2. In your opinion, how can the swine industry, the public health community, and other government agencies work together to prevent and detect any zoonotic influenza strains that may be a threat to public health?
Swine influenza as a zoonotic disease
1. In your province, how much of a threat are swine influenza viruses to human health?
2. If swine influenza viruses are regarded as a threat to humans, what measures has public health taken to reduce this threat?
3. What further measures could be taken to reduce the threat?
4. What could other government agencies do to help reduce the threat of zoonotic influenza infection at the swine-human interface?
5. In the event of a case of human infection with a swine influenza virus, what would be the public health response?
6. In the event of a case of human infection with swine influenza would the swine in contact with the infected person be sampled and tested for influenza viruses?
7. What legal or regulatory tools are available to public health to conduct swinesampling for influenza viruses?
8. Under what conditions could these tools be used?
Novel emergent potentially pandemic zoonotic influenza at the swine-human interface
1. Is the emergence of a novel potentially pandemic zoonotic influenza virus considered to be a threat in your province?
2. Is the seasonal flu vaccine free in your province?
3. Are there specific programs to encourage swine workers and their families to be vaccinated with seasonal flu vaccine?
4. Are there any other programs aimed at prevention of interspecies transmission of influenza virtues in your province?
5. Are temporary foreign workers (i.e., agricultural workers) eligible for these programs?
6. If there are specific programs, please describe the responsible stakeholders.
7. In the event of a case of human infection with a novel emergent potentially pandemic zoonotic influenza virus, what would be the public health response?
8. In the event of a case of human infection with novel emergent potentially pandemic zoonotic influenza virus would the swine in contact with the infected person be sampled and tested for influenza viruses?
9. If not already stated or different from a case of human infection with SIV, what legal or regulatory tools are available to public health to conduct swine sampling for influenza viruses?
10. Under what conditions could these tools be used?
Surveillance
1. What surveillance measures exist currently to detect swine influenza virus infections in humans in your province?
2. Is the question about animal contact part of routine influenza surveillance?
3. Under what conditions is the question about animal contact asked of a patient with ILI?
4. If the question about animal contact is not asked at the outset of influenza surveillance, at what stage of the investigation would the question about animal contact be asked?
5. Would surveillance of swine influenza virus in swine be a benefit to surveillance of human influenza virus?
6. If yes, then how could surveillance of influenza in swine be conducted to maximize the benefit.
7. If not already stated, how could the information from surveillance of influenza in swine be used to benefit public health in your province? 8. Who should be responsible for this surveillance?
9. Who should be responsible for the cost of this surveillance?
10. Are there any specific ethical issues regarding surveillance of zoonotic influenza virus?
Communication
1. Does the CMOH have regular contact with the Chief Veterinary Officer of your province regarding zoonotic disease?
2. What form of communication does this exchange take?
3. Do human health labs and animal health laboratories have any exchange of information regarding zoonotic diseases?
4. What form of communication does this exchange take?
Closing questions
1. Are there any other issues that have not been considered by these questions?
2. Are there any specific actions that the federal level of government agencies could take to help prevent and detect zoonotic influenza virus infections in your province?
3. Are there any other persons that you recommend that I talk to?
4. Would you like a copy of this report when it is complete?
Industry Swine Health Groups
The goals of this survey are:
1. To establish the current state of surveillance for zoonotic influenza viruses at the swine-human interface.
2. To clarify the utility of and most effective and efficient methods of surveillance of swine, and swine workers, on the farm and/or at abattoirs and other venues at the human-swine interface for potential zoonotic influenza viruses.
3. To clarify the best methods of interspecies virus transmission prevention and interruption. It is hoped that the information provided will form the basis for knowledge, trust, dialogue, and collaboration among the stakeholders involved with a potential zoonotic pandemic influenza virus.
General Questions: Healthy animals – Healthy people
1. In your opinion, how can swine producers and the public health community work together to prevent and detect any zoonotic influenza strains that may be a threat to swine health?
2. In your opinion, how can swine producers and the public health community work together to prevent and detect any zoonotic influenza strains that may be a threat to public health?
Swine influenza virus (SIV) in swine
1. How big of a problem is swine influenza to the industry?
2. What are the impacts of swine influenza on the industry domestically?
3. What are the impacts of swine influenza on industry exports?
4. In your opinion how widely are SIV vaccines used in the swine industry? 5. Under what conditions would SIV vaccines be used?
6. If SIV vaccines are used, in which animals are they most likely to be given?
7. If SIV vaccines are used, at what stage of production are they most likely to be given?
Swine influenza as a zoonotic disease?
1. How much of a threat are human influenza viruses to swine?
2. If human influenza virus is regarded as a threat to swine, what measures has the swine industry taken to reduce this threat?
3. What further measures could be taken to reduce the threat?
4. What can government agencies do to help the industry reduce this threat?
5. In your opinion, how much of a threat are swine influenza viruses to human health?
6. If swine influenza viruses are regarded as a threat to humans, what measures has the swine industry taken to reduce this threat?
7. What further measure could be taken to reduce the threat? 8. What can government agencies do to help the industry reduce the threat?
Surveillance of swine influenza viruses (SIV) in swine
1. Would surveillance of SIV strains in swine be a benefit to the industry?
2. Under what circumstances would surveillance of SIV be a benefit to the industry?
3. What impacts would SIV surveillance in swine have on the industry?
4. What surveillance methods would minimize any negative impacts that surveillance could have on the industry?
5. What surveillance methods may enhance the benefits of SIV surveillance in swine?
6. Which stakeholders should take the responsibility for SIV surveillance?
7. Would free SIV testing result in more sample submissions?
8. Would the option of anonymous sample submission result in more sample submissions?
9. Is there anything that government agencies could do to help the industry with surveillance of SIV in swine?
10. Does the swine industry currently have partnerships with swine health researchers to conduct SIV surveillance on a regional basis in Canada?
11. How could swine researchers and the industry work together to improve the surveillance of SIV in swine?
Surveillance of zoonotic influenza virus at the swine-human interface
1. In your experience what has been the public health response to cases of swine influenza in a human?
2. What has been the impact on the industry from the public health response?
3. What public health response would be the most appropriate?
4. Would the industry support targeted surveillance of influenza at the swine-human interface if a case of swine influenza occurred in a human? (targeted surveillance could involve testing of swine in direct contact with the person diagnosed with swine influenza virus)
5. What conditions would need to exist for the industry to broadly support targeted surveillance as described above?
6. What would the provincial agriculture department’s response be in the event of a human becoming infected with a swine influenza virus?
7. What impact would this response have on the industry?
Additional questions
Are you familiar with the USDA National Surveillance Plan for Swine Influenza Virus? (The monitoring plan is comprised of laboratory surveillance of pigs exhibiting ILI either on farms or in places of comingling such as auctions or fairs AND surveillance of pigs that are epidemiologically linked to a human with confirmed SIV).
1. Would a similar monitoring plan work in Canada? Please explain.
2. Would a similar monitoring plan work if it was expanded to include swine that are epidemiologically linked to a human with any type of influenza infection, not just SIV?
3. Do you think that the federal level of government should do anything particular to reduce the threat of zoonotic influenza at the swine-human interface?
4. Are there any other considerations that have not been covered by these questions?
5. Are there other industry groups or persons that you think I should talk to?
6. Would you like a copy of the final report when it is complete?
