COMMENTARY
Heejune Chang
MD MHP CCMF FRCPC
Dr. Heejune Chang is a medical officer of health with the Winnipeg Regional Health Authority in Manitoba.
Bold words appear in the Glossary.
Background
As another flu season approaches, public health officials look both to new surveillance data and to lessons from recent seasons. Last winter, resurgence of the pandemic influenza A (H1N1) strain, (henceforth referred to as A(H1N1)pdm09), raised concerns for the effectiveness of the 2013/14 seasonal influenza vaccines.1 Given that the strain may re-emerge in a subsequent season, vaccine effectiveness against this, as well other emerging strains, remains an important area of inquiry. According to Canada’s National Advisory Committee on Immunization (NACI), annual vaccination against influenza remains the best available strategy for reducing the risk of serious illness and death due to influenza, and for reducing the impact of influenza outbreaks on the health care system and the wider economy.2 Yet, challenges in obtaining timely and valid evidence concerning the effectiveness of influenza vaccines remain a significant barrier to uptake of this information by public health practitioners.
Unlike other vaccines used in public health practice, seasonal influenza vaccines are reformulated annually to keep pace with the constantly changing influenza virus. Each year the World Health Organization (WHO) recommends vaccine strains for the northern and southern hemispheres based on currently circulating and emerging influenza virus strains. Seasonal influenza vaccine effectiveness largely depends on how well the vaccine induces immunogenicity in its target population as a consequence of how well the WHO-recommended vaccine strains antigenically match circulating viruses. The constant change in vaccine composition and the need to manufacture, distribute and administer the vaccine to millions of people in a short time makes it difficult to evaluate the field effectiveness of these vaccines using randomized clinical trials.
COMMENT
The seasonal influenza vaccine undergoes a two times per year revision cycle that other vaccines do not. Influenza VE studies done at the mid-season make use of the extensive influenza surveillance systems that exist in many nations. They can provide information that will help inform the prevention and control of influenza by public health for the remainder of a given season for national, regional and local levels of planning. This is particularly important in seasons with high rates of influenza activity. In some seasons it may be appropriate to consider augmenting vaccination efforts while in other seasons resources might be directed to other modes of prevention and control. As such, these studies are meant to be timely. The timing of such studies can also inform public health in other countries where the influenza season may have just started or may be about to start. And, VE studies contribute to forthcoming WHO vaccine composition deliberations every year.
Currently, seasonal influenza vaccines are approved for public use on the basis of small immunogenicity studies, although it is far from clear whether the results of these studies are predictive of vaccine effectiveness in the field. Several national and supranational influenza surveillance networks have developed sufficient capacity to gather enough data to provide reasonably reliable interim (mid-season) vaccine effectiveness estimates. Historically, these estimates were found to be reasonably accurate when compared with post-season estimates based on data from the entire flu season, making mid-season assessments of influenza vaccine effectiveness useful to public health practitioners.1,3,4
Objective
The objectives of this review are to describe the salient epidemiological features of the 2013/14 influenza season and summarize the mid-season evidence on vaccine effectiveness of the 2013/14 influenza vaccines. As well, the review includes information on the effectiveness of A(H1N1)pdm09 vaccines used during the 2009 pandemic and in the seasons that followed the pandemic as it may provide valuable insights into the epidemiology and control of influenza A(H1N1)pdm09 in subsequent seasons.
COMMENT
This systematic review brought together the results of all the interim VE influenza studies to date at the time it was conducted. A well done systematic review of mid-season influenza VE studies combines identifying, appraising and synthesizing the best current evidence. Systematic reviews save public health and clinical practitioners the time spent gathering and reading through studies separately and so reviews such as this one could be timely tools during the influenza season. They are also meant to be stronger sources of summary evidence through combining several studies in a single report.
Methods
This review focused on peer-reviewed, published reports of interim estimates of the effectiveness of the 2013/14 influenza vaccine. The electronic databases of Scopus, Medline, EMBASE and Google Scholar were searched for articles on influenza vaccine effectiveness published between January 1, 2014 and March 31st. The literature search was limited to articles published in English. Bibliographies of identified publications were manually searched for additional citations. Studies were included in the review if the 2013/14 influenza vaccine was the exposure of interest and if the outcome examined was any of: influenza-like illness (ILI), laboratory confirmed influenza, hospitalization, or death. Information extracted from all identified studies included: study region and recruitment dates, immunization campaign start date, study design, study population, outcomes, confounders adjusted for in analyses, as well as estimates of vaccine effectiveness and associated confidence intervals (95%CI).
COMMENT
Many factors influence the outcomes of a VE study. Obviously, it will depend on how well matched the circulating viruses are with those included in the current vaccine. The study design should always be taken into consideration when interpreting results. Factors influencing VE include when and where the study is done, the host factors of the study population, and the definition of infection used. Public health and clinical practitioners need to keep in mind that VE studies are usually observational studies and may be subject to bias or confounding.
Epidemiologic features of 2013/14 influenza at mid-season
At approximately mid-season, global surveillance data indicated that A(H1N1)pdm09 was the predominant circulating influenza strain in North America, Europe, Australia and Eastern Asia. Since the emergence and spread of the A(H1N1)pdm09 as a novel virus in the Spring of 2009, its activity was minimal in most temperate countries until the Fall of 2013. For instance, during the 2012/13 influenza season, influenza A(H3N2) was the predominant circulating virus, representing over 90% of typed strains in the USA and Canada.5,6 However, near mid-season in 2013/14, more than 90% of detected influenza strains in the United States and Canada were A(H1N1)pdm09-like.1 In other parts of the world, regional and widespread A(H1N1)pdm09 activity was noted in Australia, China, Japan, Egypt and several European countries including France, Greece, Portugal and Switzerland.7
In Canada, an analysis conducted by the National Collaborating Centre for Infectious Diseases with data from mid-January suggested that the 2013/14 season might be more “severe” (in terms of rates of reported hospitalizations, intensive care admissions and deaths) than recent seasons.8 (According to a mid-August FluWatch report, by the end of the 2013/14 season, there were more hospitalizations than the previous year, and a greater proportion of those hospitalized were admitted to ICU, but a similar number of deaths were reported.) By late March, there were 3,332 reported hospitalizations and 182 reported deaths in Canada. Adults aged 20-64 were apparently most likely to be diagnosed, hospitalized and to die from influenza infection.5 Among adult patients requiring intensive care, about 85% reported having at least one comorbid condition; the prevalence of comorbidities and an increase in the median age of severe, hospitalized cases of influenza indicated potential changes in epidemiology compared with the 2009 pandemic.5,9 Unlike what was observed in Canada, interim data from several European countries suggested that outcomes were less severe there in the 2013/14 season than in the previous influenza season.10
Virus characterization data from various countries, including Canada, indicated that near mid-season the circulating A(H1N1)pdm09 virus remained genetically and antigenically similar to the A/California/07/2009 virus that caused the 2009 pandemic. This is the same virus that has been the WHO-recommended H1N1 reference vaccine strain in all seasons since 2009, including the 2013/14 season1,10,11 Furthermore, the majority of circulating viruses were found to be susceptible to oseltamivir and zanamivir.5,6 These data did not suggest that antigenic drift in the circulating virus strain played an important role in the re-emergence of A(H1N1)pdm09 in the 2013/14 season. Therefore, it became necessary to consider other factors, either those related to the epidemiology of A(H1N1)pdm09, such as a high number of susceptible individuals or waning immunity, or vaccine effectiveness.1 As a context for evaluating the effectiveness of the 2013/14 vaccine, consideration of the effectiveness of A(H1N1)pdm09 vaccines used during and since the 2009 pandemic follows.
The A(H1N1)pdm09 virus
The A(H1N1)pdm09 was first identified in the spring of 2009 following reports of severe influenza-like illnesses (ILI) in Mexico and the Southern USA.12 By the time the WHO declared a pandemic on June 11, 2009, the novel virus had spread to more than 74 countries. As with previous pandemics, the 2009 pandemic was characterized by unusually higher rates of morbidity in previously healthy, young adults. Unlike children and younger adults, those older than 60 years were more likely to have cross-reactive antibodies against the novel virus and, therefore, were less likely to be infected.12,13 On the other hand, children under the age of 5 years were more likely to be hospitalized and older adults (aged 65+) were more likely to die if infected, compared to individuals in other age groups.14 Besides age, the presence of comorbidities, obesity, pregnancy and belonging to an Aboriginal population were also identified as risk factors for more severe outcomes.12,15
Efficacy and effectiveness of monovalent A(H1N1)pdm09 vaccines
A total of 26 vaccine manufacturers developed monovalent A(H1N1)pdm09 vaccines for use during the pandemic.12 In Canada, GlaxoSmithKline (GSK) produced an ASO3–adjuvanted vaccine as well as unadjuvanted vaccines. Both contained the A(H1N1)pdm09 antigen derived from the influenza A/California/7/2009 strain, as was recommended by the WHO. Excellent immune responses following even one dose of the monovalent split and subunit inactivated pandemic H1N1 vaccines, with or without adjuvant, were documented in several immunogenicity trials conducted around the time the vaccine was introduced.16,17 Generally, vaccines with oil-in-water adjuvants were more immunogenic than unadjuvanted vaccines.17Even for the unadjuvanted vaccines, most comparable with the seasonal influenza vaccines, presumably protective levels of antibodies (hemagglutination-inhibiting antibody titres >1:40) were achieved among healthy adults and older children.
Studies from the 2009 pandemic provide a range of estimates of the effectiveness of the monovalent vaccine for preventing infections and hospitalizations due to laboratory-confirmed A(H1N1)pdm09, and suggest that some age groups may be inadequately protected. In a systematic review of five observational studies, the median vaccine effectiveness for the monovalent A(H1N1)pdm09 was estimated at 69%.18 Our own review of 46 studies, all conducted to assess the effectiveness of the monovalent vaccine during the pandemic, suggested that the vaccine was around 80% effective against laboratory-confirmed A(H1N1)pdm09 infections, with estimates from these studies ranging from 46 to 100% (unpublished data). Estimates of vaccine effectiveness against hospitalizations due to laboratory-confirmed A(H1N1)pdm09 infections ranged from 19 to 99% with a pooled vaccine effectiveness of about 65%. Generally, the monovalent vaccines were most effective among children. As with immunogenicity trials, the adjuvanted vaccines were more effective than unadjuvanted vaccines (pooled vaccine effectiveness around 60%). Unadjuvanted vaccines were more effective in preventing laboratory confirmed influenza infections among school-age children and young adults than among older adults. For instance, in one study, one dose of an inactivated vaccine was about 77% effective in preventing laboratory-confirmed A(H1N1)pdm09 infection among 10–49 year-olds, but only 22% effective among those aged 50 years or older (unpublished data).19 In another study, an inactivated vaccine was about 58% effective in preventing A(H1N1)pdm09 infection among 5-14 year-olds.20 In both studies, the live attenuated vaccines were more effective than inactivated vaccine among children, around 80%.
Effectiveness of seasonal influenza vaccines in post-pandemic seasons
Following the pandemic, there was a significant decrease in A(H1N1)pdm09 virus circulation and detection. Subsequent seasons in both the northern and southern hemispheres were dominated by influenza A (H3N2) and influenza B subtypes. Starting in the 2010/11 season, the A/California/07/2009 strain has been recommended for inclusion in every year’s seasonal vaccine for both hemispheres.10 The effectiveness of these vaccines against laboratory-confirmed A(H1N1)pdm09 infection was extensively evaluated in 2010/11. There were fewer studies in subsequent seasons, but their results were similar to the evaluations conducted during the pandemic. Generally, estimates of overall vaccine effectiveness ranged from 55 to 80%, depending on age group, season and region, and were highest among children and younger adults and lowest among older (>50) adults.21-23 For instance, a study based on data from the Canadian Sentinel Physician Network showed that in the 2010/11 season, the vaccine was about 65% effective in preventing A(H1N1)pdm09 infection among 20-49 year-olds and about 30% effective among those 50 years or older.23 In the 2012/13 season, which in Canada was dominated by an H3N2 subtype, overall vaccine effectiveness against A(H1N1)pdm09 was about 80%.22
Similar estimates were reported by studies from the USA and Australia with vaccine effectiveness estimates ranging from 66% to 79%.24-26 Studies from Europe have also suggested that the 2010/11 seasonal influenza vaccine conferred significant protection against A(H1N1)pdm09 infection. For instance, a study based on the multicentre European influenza surveillance network (I-MOVE) reported a pooled estimate of vaccine effectiveness against A(H1N1)pdm09 infection of 59%.27
Effectiveness of 2013-2014 seasonal influenza vaccines
Following the WHO recommendations, the 2013/2014 seasonal trivalent influenza vaccine for the northern hemisphere contained an A/California/7/2009 (H1N1)pdm09-like virus strain, an A/Texas/50/2012 (H3N2)-like virus strain, and a B/Massachusetts/2/2012-like virus strain.10 Eight types of seasonal influenza vaccines were authorized for use in Canada during the 2013/2014 season. Seven of the vaccines were trivalent inactivated vaccines and one was a live attenuated vaccine. (Quadrivalent influenza vaccines were not yet authorized for use in Canada during the 2013/14 season.)
At the time of writing, a limited number of published epidemiological studies conducted in Canada,1,9 the US,28 and Spain,11,29 had evaluated the mid-season effectiveness of the 2013/14 seasonal influenza vaccine. Characteristics of these studies are summarized in Table 1. All were test-negative case-control studies whereby patients presenting to medical care with symptoms of ILI or other acute respiratory illnesses are classified as cases if they test positive for influenza by PCR or culture, and controls if they test negative. Therefore, the outcome assessed in all these studies was medically attended laboratory confirmed infection with influenza or an influenza subtype. Two studies included patients admitted to hospitals.9,11 All studies, but one,9 included patients seen as outpatients. No studies examined vaccine effectiveness against severity of outcomes such as hospitalization, ICU admission or death due to influenza.
COMMENT
Most interim VE studies, such as those summarized in this systematic review, use the ‘test negative’ method: Patients presenting with ILI within the sentinel physician system provide a study population. This tested population allows for comparison of test positives versus test negatives and the rate of vaccination in each group. What this case control style method does not tell us is how well that influenza infection was prevented in the population as a whole. It also does not tell us how effective vaccination was for prevention of severe outcomes e.g. death, hospitalization or outbreaks.
Table 2 summarizes estimates of the effectiveness of the 2013/14 seasonal influenza vaccines from the mid-season studies. While vaccine effectiveness estimates change over the course of an influenza season as additional data become available or if there is a change in circulating viruses late in the season, this has not been the experience during previous influenza seasons in Canada or elsewhere.1,3,4
A test-negative case-control study conducted in five Canadian provinces (British Columbia, Alberta, Ontario, Quebec, and Manitoba) involved patients, predominately 20-49 years old, who visited a community-based sentinel physician with ILI symptoms.1 The analysis included 792 specimens collected from patients presenting between 1 November 2013 (week 44) and 23 January 2014 (week 4); 41% of the specimens were positive for influenza with 90% of typed specimens testing positive for A(H1N1)pdm09. Overall, a greater proportion of controls (32%) reported the receipt of a 2013/14 seasonal influenza vaccine compared to cases (12%). Vaccine types were not specified, but in Canada, adults almost exclusively receive the intramuscular unadjuvanted split-virion trivalent seasonal vaccines.9 Vaccine effectiveness, adjusted for age, comorbidity, province and epidemic week, was 74% (95% CI: 58 – 83%) in preventing laboratory-confirmed A(H1N1)pdm09 infection (Table 2). This estimate is in the upper range of recent seasons’ estimates reported from the same Canadian sentinel surveillance network, with vaccine effectiveness against A(H1N1)pdm09 ranging from 59 to 80%.30-32 The study did not have sufficient power to estimate vaccine effectiveness among children or seniors. A(H1N1)pdm09 viruses characterized from a subset of the study population were antigenically and genetically similar to the WHO-recommended A/California/7/2009 reference vaccine strain.
Another Canadian team reported interim vaccine effectiveness estimates using data from the Canadian Serious Outcomes Surveillance (SOS) Network which includes 40 adult acute care hospitals from seven Canadian provinces (New Brunswick, Nova Scotia, Quebec, Ontario, Manitoba, Alberta, and British Columbia).9 The population of this test-negative case-control study comprised adults (aged 16 years or older) hospitalized between November 15, 2013 and February 8, 2014 with a diagnosis of ILI, pneumonia or other respiratory infections, exacerbation of chronic obstructive respiratory disease or asthma, or unexplained sepsis. Among this older population (38% of cases and 63% of controls were 65 years or older) with high levels of comorbidity (92% had at least one pre-existing disease), 35% of cases and 62% of controls reported receiving a 2013/14 seasonal influenza vaccine. Virtually all vaccinated persons received the intramuscular split-virion trivalent vaccine. The age- and comorbidity-adjusted vaccine effectiveness against A(H1N1)pdm09 infection was 58% (38 – 72%) (Table 2). Vaccine effectiveness estimates were similar among 16-64 year-old adults (54% [95% CI: 22 – 73%]) and adults aged 65 years or older (63% [95% CI: 35 – 79%]). These estimates were somewhat lower than reported from the abovementioned Canadian community-based study.1 However, lesser effectiveness is expected as the hospitalized population was much older with a far greater proportion of persons with underlying comorbidities than the community-based study.
Another community-based test-negative case-control study used data from persons enrolled between December 2, 2013 and January 23, 2014 at one of five sentinel sites that contribute data to the U.S. Influenza Vaccine Effectiveness (Flu-VE) Network. The study provided vaccine effectiveness estimates that were generally consistent with those reported for Canada.28 The analysis included persons aged 6 months or older seeking outpatient medical care for an acute respiratory infection. Vaccine effectiveness of the 2013/14 seasonal influenza vaccines (exact type was not specified) against influenza A(H1N1)pdm09 virus was 62% (95%CI: 53 – 69%) (Table 2). Seasonal influenza vaccine was similarly effective across age groups, with a slight decrease among older (65 years or older) adults. These estimates were slightly lower than those reported from similar US studies since the 2010 influenza season, with vaccine effectiveness estimates ranging from 66 to 77%.24,25,33
On the other hand, two reports from Spain11,29 indicated much lower vaccine effectiveness estimates than those reported by North American studies (Table 2).1,9,28 In Spain, the 2013/14 season was not as intense or as dominated by A(H1N1)pdm09 as in North America; only 60% of typed isolates were A(H1N1)pdm09. Interim vaccine effectiveness estimates from the nationwide Spanish Influenza Sentinel Surveillance System was 33% (95% CI: −33 – 67) against medically attended A(H1N1)pdm09 infection.29 Very similar estimates were reported for “high-risk” populations (36% [95%CI:-64-75]). The vaccine used in Spain was a split-virion inactivated unadjuvanted trivalent vaccine. Similar results were also obtained from analyses limited to data from the Navarre region (VE: 40%, 95% CI: -12 – 68%).11 However, in the Navarre study, clinically significant differences in vaccine effectiveness were found between persons younger than 65 years (59%; 95% CI: 4 to 83) and those aged 65 or more (4%; 95% CI: −162 to 65), although the imprecise nature of these estimates, as indicated by extremely wide confidence intervals, preclude any firm conclusions.11
It is not clear why Spain had lower vaccine effectiveness estimates. Characterized isolates were mostly antigenically and genetically similar to the vaccine reference strain. In Spain, similar suboptimal protection against A(H1N1)pdm09 virus was also reported during the 2010/11 season, with vaccine effectiveness ranging from 46% (95% CI: 0 – 72)34 to 49% (95%CI: 3 – 73%).35 Surprisingly, higher vaccine effectiveness estimates were reported among high-risk populations, ranging from 47% to 63%.34-37 The test-negative controls in this season’s study had a higher percentage of high-risk persons (36%) compared to A(H1N1)pdm09 cases (23%) which may explain the higher vaccine uptake (17%) among them compared to the cases (11%).29
Analysis
After several seasons of low disease activity, a significant surge in influenza A(H1N1)pdm09 activity was observed worldwide in the first half of the 2013/14 season. Concerns were raised about the effectiveness of 2013/14 seasonal influenza vaccine and the possibility of antigenic drift. However, in our review of interim 2013/14 vaccine effectiveness analyses from North America (and Spain) we found no evidence that the 2013/14 vaccine was less effective than similar vaccines used in previous seasons, including the monovalent vaccines used during the pandemic. Studies from North America were consistent in showing 62-74% protection against laboratory-confirmed A(H1N1)pdm09 infection. These estimates are also consistent with estimates of seasonal vaccine effectiveness against A(H1N1)pdm09 infection observed in previous seasons since the pandemic. The estimates are generally in the upper range of vaccine effectiveness estimates against infection with other influenza strains circulating before the pandemic, during seasons with a good vaccine match.38
Data on vaccine effectiveness among persons at high-risk of influenza complications were limited, but available information suggest slightly lower effectiveness among older and hospitalized persons. Relatively small sample sizes resulted in limited ability to provide precise estimates of vaccine effectiveness among different age groups. No studies examined vaccine effectiveness against hospitalization, ICU admission or death due to influenza. Despite progress achieved in measuring interim vaccine effectiveness, we are still far away from being able to measure interim vaccine effectiveness against severe illness, and among high-risk populations. Achieving this objective will require expanding and strengthening national influenza surveillance networks.
Evidence from studies reviewed in this report as well as from national surveillance data indicate that, at the time of writing, the circulating A(H1N1)pdm09 virus had remained genetically and antigenically similar to the A/California/07/2009 virus that caused the 2009 pandemic as well as the 2013/14 season’s reference vaccine strain. Gene sequencing of a convenience sample of viral isolates from the Canadian Sentinel Physician Surveillance Network found that these isolates shared > 90% of their antigenic site sequence with the vaccine strains, and very rarely had more than the same 3 antigenic site mutations seen among dominant A(H1N1)pdm09 strains in previous seasons.1 These findings support the decision of the WHO to retain the A/California/07/2009-like strain as the H1N1 component in the vaccines for this current (2014/15) season.7 In fact, these data, along with laboratory and global surveillance data, were used to reach that decision.
Taken together, this information suggests that the resurgence of an unchanged pandemic virus in the 2013/14 season was likely related to lower levels of population immunity rather than to antigenic changes resulting in vaccine strain mismatch. This conclusion appears consistent with results of a seroprevalence survey conducted in British Columbia, reported by Skowronski and colleagues in a (January 2014) ProMED-mail post. The lowest levels of seroprotection were found among “children under 5 years of age (most of whom were not alive during the 2009 pandemic) and the highest levels (≥60 %) appeared in school-age children and those 70 years or older.”39 The results point to the importance of considering agent-host interaction factors in explanation of A(H1N1)pdm09 epidemiology, including the presence of pre-existing antibodies among various age groups.
Comment
Because A(H1N1)pdm09 was the dominant influenza strain in 2013/14 influenza season, the interim studies that year also contribute information to public health knowledge of this influenza virus. This systematic review of interim VE studies for the Northern hemisphere suggested the VE for A(H1N1)pdm09 was reasonably effective. These VE results were consistent with antigenic testing in 2013-14 of the A(H1N1)pdm09 virus—which showed that it had remained relatively stable so far from its 2009 form—and with studies that showed that the vaccine strain of A(H1N1)pdm09 continued to be well matched to the circulating strain.
References
1. Skowronski DMC, C.; N.Z.; De Serres, G.; Dickinson, J.A.; Winter, A-L.; Fonseca, K.; Gubbay, J.B.; Charest, H.; Petric, M.; Krajden, M.; Mahmud, S.M.; Van Caseele, P.; Kwindt, T.L.; Eshaghi, A.; Li, Y.; Bastien, N.; Li, Y. Interim estimates of 2013/14 vaccine effectiveness against influenza A(H1N1)pdm09 from Canada’s sentinel surveillance network, January 2014. Eurosurveillance 2014;19.
2. National Advisory Committee on Immunization (NACI). Statement on Seasonal Trivalent Inactivated Influenza Vaccine (TIV) for 2009-2010. CCDR 2009;35.
3. Sullivan SG, Kelly H. Late season interim estimates of influenza vaccine effectiveness reliably predict end of season estimates in Victoria, Australia, 2007 to 2012. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2013;18:20605.
4. Belongia EA, Kieke BA, Donahue JG, et al. Influenza vaccine effectiveness in Wisconsin during the 2007-08 season: comparison of interim and final results. Vaccine 2011;29:6558-63.
5. Weekly reports 2013-2014 season. PHAC, 2014. (Accessed March 23, 2014, at http://www.phac-aspc.gc.ca/fluwatch/13-14/index-eng.php.)
6. FluView. Past Weekly Surveillance Reports. CDC, 2014. 23 March 2014 at http://www.cdc.gov/flu/weekly/pastreports.htm.)
7. Recommended composition of influenza virus vaccines for use in the 2013-2014 northern hemisphere influenza season. February 2013. (Accessed 23 March 2014, at http://www.who.int/influenza/vaccines/virus/recommendations/2013_14_north/en/.)
8. RAPID REVIEW: Influenza Season 2013-2014. Ten Questions and Answers for Canadian Public Health Practitioners. NCCID, 2014. (Accessed 23 March 2014, at https://www.nccid.ca/files/Influenza/ENG-H1N1_Rapid_Review.pdf.)
9. McNeil SAS, V.; Andrew, M.; Hatchette, T.F.; Leblanc, J.; Ambrose, A.; Boivin, G.; Bowie, W.R.; Diaz-Mitoma, F.; ElSherif, M.; Green, K.; Haguinet, F.; Halperin, S.; Ibarguchi, B.; Katz, K.; Langley, J.M.; Lagacé-Wiens, P.; Light, B.; Loeb, M.; McElhaney, J.; MacKinnon-Cameron, D.; McCarthy, A.E.; Poirier, M.; Powis, J.; Richardson, D.; Semret, M.; Smith, S.; Smyth, D.; Stiver, G.; Trottier, S.; Valiquette, L.; Webster, D.; Ye, L.; McGeer, A.; on behalf of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) Serious Outcomes Surveillance Network; on behalf of the Toronto Invasive Bacterial Diseases Network (TIBDN). Interim estimtes of 2013/14 influenza clinical severity and vaccine effectiveness in the prevention of laboratory-confirmed infleunza-related hospitalisation, Canada, February 2014. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2014;19.
10. World Health Organization: Recommended composition of influenza virus vaccines for use in the 2013-2014 northern hemisphere influenza season. February 2013.
11. Castilla JM-B, I.; Navascues, A.; Fernandez-Alonso, M.; Reina, G.; Guevara, M.; Chamorro, J.; Ortega, M.T.; Albeniz, E.; Pozo, F.; Ezpeleta, C.; Primary Health Care Sentinel Network, Network for Influenza Surveillance in hospitals of Navarre. Vaccine effectiveness in preventing laboratory-confirmed influenza in Navarre, Spain: 2013/14 mid-season analysis. Eurosurveillance 2014;19.
12. Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: A review. Vaccine 2010;28:4895-902.
13. Katz, Hancock, Veguilla, et al. Serum Cross-Reactive Antibody Response to a Novel Influenza A (H1N1) Virus After Vaccination with Seasonal Influenza Vaccine. MMWR 2009; 58:521-4.
14. Donaldson LJ, Rutter PD, Ellis BM, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2009;339:b5213.
15. Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009;14.
16. Yin JK, Khandaker G, Rashid H, Heron L, Ridda I, Booy R. Immunogenicity and safety of pandemic influenza A (H1N1) 2009 vaccine: systematic review and meta-analysis. Influenza Other Respir Viruses 2011;5:299-305.
17. Manzoli L, De Vito C, Salanti G, D’Addario M, Villari P, Ioannidis JPA. Meta-Analysis of the Immunogenicity and Tolerability of Pandemic Influenza A 2009 (H1N1) Vaccines. PLoS ONE 2011;6:e24384.
18. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. The Lancet Infectious Diseases 2012;12:36-44.
19. Griffin MR, Monto AS, Belongia EA, et al. Effectiveness of Non-Adjuvanted Pandemic Influenza A Vaccines for Preventing Pandemic Influenza Acute Respiratory Illness Visits in 4 U.S. Communities. PLoS ONE 2011;6:e23085.
20. Uzicanin A, Thompson M, Smith P, et al. Effectiveness of 1 Dose of Influenza A (H1N1) 2009 Monovalent Vaccines in Preventing Reverse-Transcription Polymerase Chain Reaction–Confirmed H1N1 Infection Among School-Aged Children in Maine. J Infect Dis 2012;206:1059-68.
21. Kissling E, Valenciano M, Cohen JM, et al. I-MOVE Multi-Centre Case Control Study 2010-11: Overall and Stratified Estimates of Influenza Vaccine Effectiveness in Europe. PLoS ONE 2011;6:e27622.
22. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011-12 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014.
23. Skowronski DM, Janjua NZ, De Serres G, et al. A Sentinel Platform to Evaluate Influenza Vaccine Effectiveness and New Variant Circulation, Canada 2010–2011 Season. Clin Infect Dis 2012;55:332-42.
24. Bateman AK, B.; Irving, S.; Meece, J.; Shay, D.; Belongia, E. Effectiveness of Monovalent 2009 PandemicInfluenza A Virus Subtype H1N1 and 2010–2011 Trivalent Inactivated Influenza Vaccines in Wisconsin During the 2010–2011 Influenza Season. The Journal of Infectious Diseases 2013;207:1262-9.
25. Treanor JT, H.K.; Ohmit, S.; Coleman, L.; Thompson, M.; Cheng, P.Y.; Petrie, J.; Lofthus, G.; Meece, J.; Williams, J.; Berman, L.; Hall, C.; Monto, A.; Griffin, M.; Belongia, E.; Shay, D. for the US Flu-VE network. Effectiveness of Seasonal Influenza Vaccines in the United States During a Season With Circulation of All Three Vaccine Strains. Clinical Infectious Diseases 2012;55:951-9.
26. Fielding JE, Grant KA, Garcia K, Kelly HA. Effectiveness of seasonal influenza vaccine against pandemic (H1N1) 2009 virus, Australia, 2010. Emerging infectious diseases 2011;17:1181-7.
27. Kissling EV, M.; Cohen, J.M.; Oroszi, B.; Barret, A.S.; Rizzo, C.; Stefanoff, P.; Nunes, B.; Pitigoi, D.; Larrauri, A.; Daviaud, I.; Horvath, J.K.; O’Donell, J.; Seyler, T.; Paradowska-Stankiewicz, I.A.; Pechirra, P.; Ivanuvic, A.E.; Jimenez-Jorge, S.; Savulescu, C.; Ciancio, B.C.; Moren, A. . I-MOVE Multi-Centre Case Control Study 2010-11: Overall and Stratified Estimates of Influenza Vaccine Effectiveness in Europe. PLoS ONE 2011;6.
28. Flannery B, Thaker SN, Clippard J, et al. Interim estimates of 2013-14 seasonal influenza vaccine effectiveness – United States, february 2014. MMWR Morbidity and mortality weekly report 2014;63:137-42.
29. Jiménez-Jorge SP, F.; de Mateo, S.; Delgado-Sanz, C.; Casas, I.; García-Cenoz, M.; Castilla, J.; Sancho, R.; Etxebarriarteun-Aranzabal, L.; Quinones, C.; Martínez, E.; Vega, T.; Garcia, A.; Giménez, J.; Vanrell, J.M.; Castrillejo, D.; Larrauri, A.; on behalf of the Spanish Influenza Sentinel Surveillance System (SISS),. Influenza vaccine effectiveness in Spain 2013/14: subtype-specific early estimates using the cycEVA study. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2014;19.
30. Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010-2011 season. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2012;55:332-42.
31. Skowronski DMJ, N.Z.; De Serres, G.; Dickinson, J.A.; Winter, A-L.; Mahmud, S.M.; Sabaiduc, S.; Gubbay, J.B.; Charest, H.; Petric, M.; Van Caseele, P.; Kwindt, T.L.; Krajden, M.; Eshaghi, A.; Li, Y. Interim estimates of influenza vaccine effectiveness in 2012/13 from Canada’s sentinel surveillance network, January 2013. Eurosurveillance 2013;2013:5.
32. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011-12 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014.
33. Ohmit ST, M.; Petrie, J.; Thaker, S.; Jackson, M.; Belongia, E.; Zimmerman, R.; Gaglani, M.; Lomerato, L.; Spencer, S.; Jackson, L.; Meece, J.; Nowalk, M.P.; Song, J.; Zervos, M.; Cheng, P.Y.; Rinaldo, C.; Clipper, L.; Shay, D.; Piedra, P.; Monto, A. Influenza Vaccine Effectiveness in the 2011–2012 Season: Protection Against Each Circulating Virus and the Effect of Prior Vaccination on Estimates. Clinical Infectious Diseases 2014;58:519-27.
34. Jimenez-Jorge SS, C.; Pozo, F.; de Mateo, S.; Casas, I.; Ledesma, J.; Larrauri, A.; The cycEVA Study Team on behalf of the Spanish Influenza Sentinel Surveillance System. Effectiveness of the 2010-11 seasonal trivalent influenza vaccine in Spain: cycEVA study. Vaccine 2012;30:3595-602.
35. Savulescu CJ-J, S.; de Mateo, S.; Ledesma, S.; Pozo, F.; Casas, I.; Larrauri, A.; cycEVA Study Team. Effectiveness of the 2010/11 seasonal trivalent influenza vaccine in Spain: preliminary results of a case–control study. Eurosurveillance 2011;16.
36. Castilla JM, J.; Martinez-Artola, V.; Reina, G.; Martinez-Baz, I.; Garcia Cenoz, M.; Alvarez, N.; Irisarri, F.; Arriazu, M.; Elia, F.; Salcedo, E. . Effectiveness of trivalent seasonal and monovalent influenza A (H1N1) 2009 vaccines in population with major chronic conditions of Navarre, Spain: 2010/11 mid-season analysis. Eurosurveillance 2011;16.
37. Castilla JM-A, V.; Salcedo, E.; Martinez-Baz, I.; Garcia Cenoz, M.; Guevara, M.; Alvarez, N.; Irisarri, F.; Moran, J.; Barricarte, A.; Network for Influenza Surveillance in Hospitals of Navarre. Vaccine effectiveness in preventing influenza hospitalizations in Navarre, Spain, 2010-2011; Cohort and case-control study. Vaccine 2012;30:195-200.
38. Manzoli L, Ioannidis JP, Flacco ME, De Vito C, Villari P. Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses. Hum Vaccin Immunother 2012;8:851-62.
39. Influenza A(H1N1)pdm09 – Canada: (BC) primary data & analysis. ProMED-mail, 2014. (Accessed 23 March 2014, at http://promedmail.chip.org/pipermail/promed/2014-January/003076.html.)
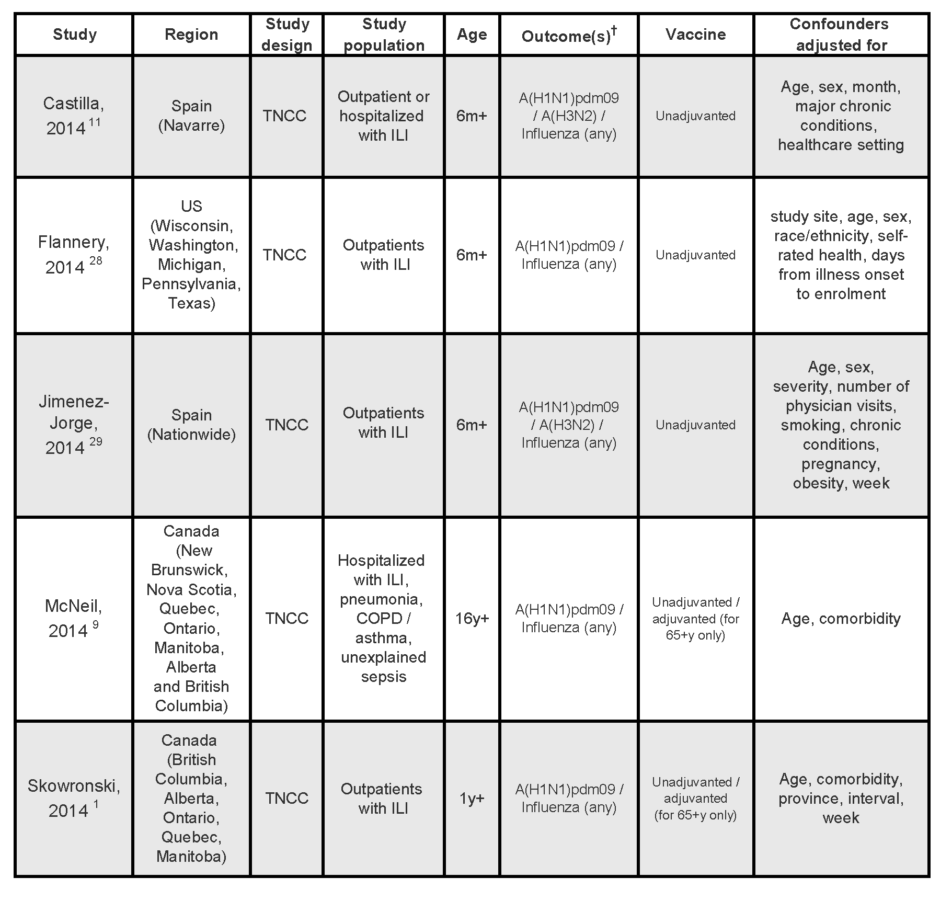
Table 1:
Characteristics of studies on mid-season effectiveness of the 2013/14 seasonal influenza vaccines
† All studies assessed medically attended laboratory-confirmed infections.
TNCC, test-negative case-control; m, months; y, years; ILI, influenza-like illness; OP, outpatient population, H, hospitalized population; COPD, chronic obstructive pulmonary disease
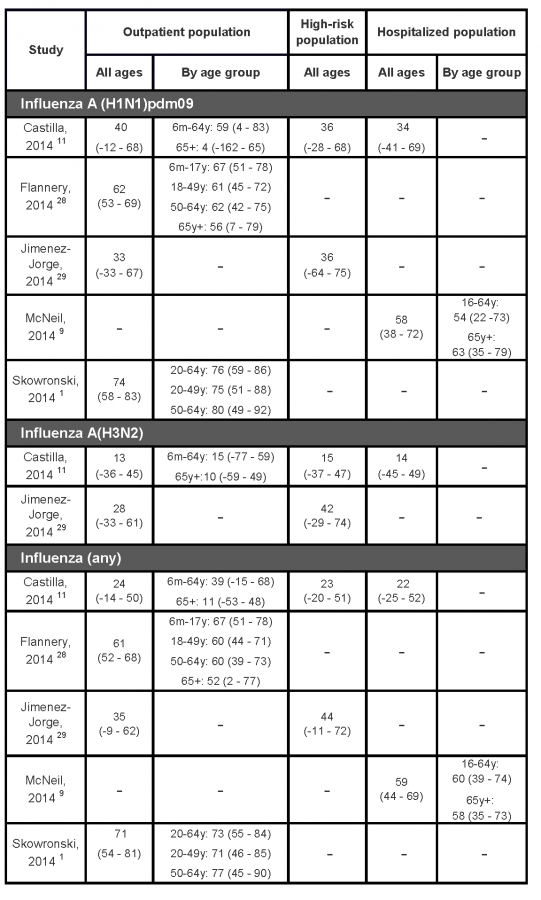
Table 2:
Interim estimates of the 2013/14 seasonal influenza vaccine effectiveness (95% confidence interval) in preventing laboratory confirmed influenza
Glossary
Detailed information on references for these definitions follow.
Adjuvant: Material added to an antigen to increase its immunogenicity (McGraw Hill). BACK
Antigen: A substance (e.g. a protein) that is capable of inducing specific immune response. Introduction of antigen may be by the invasion of infectious organisms, immunization, inhalation, ingestion, etc. (Porta, 2008). BACK
Antigenically: Adverb form of antigen (see previous).
Antigenic drift: The “evolutionary” changes that take place in the molecular structure of DNA/RNA in microorganisms during their passage from one host to another. It may be due to recombination, deletion, or insertion of genes, to point mutations, or to several of these events…. It leads to alteration (usually slow and progressive) in the antigenic composition and thus in the immunological responses of individuals and populations exposed to the microorganisms concerned (Porta, 2008). See also ‘antigenic shift’. BACK
Antigenic shift: A mutation, or sudden change in molecular structure of DNA/RNA, in microorganisms, especially viruses, that produces new strains of the microorganism. Hosts previously exposed to other strains have little or no acquired immunity. Antigenic shift if believed to be the explanation for the occurrence of strains of the influenza A virus associated with large-scale epidemic and pandemic spread (Porta, 2008). See also ‘antigenic drift’.
ASO3: AS03, or Adjuvant System 03, is the trade name for an adjuvant used in various vaccine products by GlaxoSmithKline (GSK). It contains DL-α-tocopherol (vitamin E), squalene and polysorbate 80 prepared as an oil-in-water (o/w) emulsion (Health Canada). BACK
Cross-reactive antibody: An antibody that reacts with antigens that are similar to, but different than, the specific antigens with which it originally reacted (Mosby’s Medical Dictionary, 2009). BACK
Effectiveness: A measure of the extent to which a specific intervention, procedure, regimen, or service, when deployed in the field in the usual circumstances, does what it is intended to do for a specified population (Porta, 2008). BACK
Efficacy: The extent to which a specific intervention, procedure, regimen, or service produces a beneficial result under ideal conditions; the benefit or utility to the individual or the population of the service, treatment regimen, or intervention. Ideally, the determination of efficacy is based on the results of a randomized controlled trial (Porta, 2008). BACK
Gene sequencing: Gene or DNA sequencing is the process of determining the precise order of nucleotides within a DNA molecule. It includes any method or technology that is used to determine the order of the four bases—adenine, guanine, cytosine, and thymine—in a strand of DNA. (Wikipedia) BACK
Immunogenicity: The ability of an infectious agent to induce specific immunity (Porta, 2008). BACK
Inactivated vaccine: Vaccines made by inactivating, or killing, the virus during the process of making the vaccine. Often, multiple doses are necessary to build up and/or maintain immunity. Inactivated vaccines may be given to persons with weak immune systems, who cannot receive live vaccines (CDC). BACK
Oseltamivir (Tamiflu): is an antiviral medication licensed to treat influenza in people 2 weeks of age and older. It has also be given to prevent influenza in people who are at least 1 year old, who may have been exposed to the influenza virus but who do not yet have symptoms (Stedman’s Electronic Medical Dictionary). Controversy has arisen over indications for use and effectiveness of antivirals. BACK
PCR:Polymerase chain reaction is an in vitro technique used to synthesize large quantities of specific nucleotide sequences from small amounts of DNA. It employs oligonucleotide primers complementary to specific sequences in the target gene and special heat-stable DNA polymerases. (McGraw Hill). BACK
Split-virion inactivated vaccine: An inactivated vaccine formulation derived by disrupting whole virus particles with detergents (Ellebedy & Webby, 2009). BACK
Subunit inactivated vaccine: Subunit vaccine include only parts of the virus or bacteria, or subunits, instead of the entire germ. Because these vaccines contain only the essential antigens and not all the other molecules that make up the germ, side effects are less common (CDC, Understanding How Vaccines Work). BACK
Vaccine, live attenuated: A vaccine prepared from live microorganisms or functional viruses whose disease-producing ability has been weakened but whose immunogenic properties have not (Mosby’s Medical Dictionary, 2009). BACK
Vaccine, monovalent: A vaccine directed at only one pathogen. BACK
Vaccine, quadrivalent: The quadrivalent flu vaccine is designed to protect against four different flu viruses; two influenza A viruses and two influenza B viruses (CDC, Influenza (Flu)). BACK
Vaccine, trivalent: A synthetic vaccine consisting of three inactivated influenza viruses, two different influenza type A strains and one influenza type B strain (National Cancer Institute). BACK
Virion: A complete virus particle that represents the extracellular phase of the virus life cycle; at the simplest, it consists of a protein capsid surrounding a single nucleic acid molecule (McGraw Hill). BACK
Zanamivir is an antiviral medication licensed for use as a treatment for flu symptoms caused by influenza virus in patients who have had symptoms for less than 2 days. Zanamivir has also been given to prevent influenza in people who may be exposed but who do not yet have symptoms (Stedman’s Electronic Medical Dictionary). Controversy has arisen over indications for use and effectiveness of antivirals. BACK
Glossary references:
Centres for Disease Control and Prevention (CDC). Influenza (Flu), Questions & Answers, Types of Flu Vaccines. (Accessed 15 December 2014 at: http://www.cdc.gov/flu/protect/vaccine/quadrivalent.htm).
Centers for Disease Control and Prevention (CDC). Understanding How Vaccines Work. Last reviewed February 2013. (Accessed 15 December 2014 at: http://www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-understand-color-office.pdf).
Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine 27: D65-D68. Elsevier, 2009. (Accessed 8 December 2014 at:http://download.thelancet.com/flatcontentassets/H1N1-flu/prevention/prevention-29.pdf)
Health Canada. Product Information Leaflet Arepanrix™ H1N1 AS03-Adjuvanted H1N1 Pandemic Influenza Vaccine – Version 4 approved 20 April 2010. (Accessed 15 December 2014 at: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/legislation/interimorders-arretesurgence/prodinfo-vaccin-eng.php)
McGraw Hill Education, Online Learning Centre, Microbiology, Glossary. (Accessed 8 December 2014 at: http://highered.mheducation.com/sites/0072320419/student_view0/glossary_a-f.html#).
Mosby’s Medical Dictionary, 8th edition. Elsevier, 2009.
National Cancer Institute. Drug Dictionary. (Accessed 15 December 2014 at: http://www.cancer.gov/drugdictionary?cdrid=38385).
Porta M. (Ed.) A Dictionary of Epidemiology, Fifth Edition. Edited for the International Epidemiological Association. Oxford University Press, 2008.
Stedman’s Electronic Medical Dictionary (version 6.0). (Accessed 15 December 2014 at: http://www.drugs.com).
