Introduction
National and local governments must determine priorities for health research and make decisions about investment in health systems and in health interventions in the face of limited resources, constantly increasing demands for healthcare, the development of new interventions and treatments, and increasing healthcare costs (1). Having a consistent and comparable description of the burden of diseases and the risk factors that contribute to them is important to health decision-making and planning processes (2). Summary measures of population health are popular and widely used because they provide understandable representations of complex epidemiology that can be used to develop efficient preventive strategies (1,2).
List of Abbreviations:
CDC: Center for Disease Control and Prevention
CLAMES: Classification and measurement system of functional health
DALY: Disability-adjusted life year
DW: Disability weight
GBD: Global burden of disease
HALE: Healthy life expectancy
HALY: Health-adjusted life year
HRQL: Health-related quality of life
ICD: International Statistical Classification of Diseases, Injuries and Causes of Death
ONBOIDS: Ontario Burden of Infectious Disease Study
QALY: Quality-adjusted life year WHO: World Health Organization
YERF: Year-equivalent of reduced functioning
Population health summary measures can be reported at international, national, or local levels. They have three main uses:
- To compare population health across communities and over time”;
- To provide a full picture of which diseases, injuries and risk factors contribute the most to poor health in a specific population, including identification of the most important health problems and whether they are getting better or worse over time (this is probably the most common use of summary measures of health);
- To assess which information or sources of information are missing, uncertain, or of low quality (3).
A complete and comprehensive burden of disease report must be based on a consistent set of estimates for mortality and morbidity. For this reason the Global Burden of Diseases, Injuries and Risk Factors project (GBD) aims to measure population health worldwide. In 1993, the World Bank published a report entitled Investing in Health, and developed methods to calculate the 1990 GBD. The findings of this analysis were published in 1996, including a report on the worldwide burden of disease for 107 diseases and injuries and 10 risk factors (2,4). The World Health Organization (WHO) has subsequently produced estimates of GBD for the years 1999, 2000, Introduction 2001, and 2004, but those reports analyzed of a subset of health conditions (pathological conditions resulting from a disease or trauma) and are consequently considered partial GBD reports (2). Since 2000, the GBD has been calculated by estimating disability and mortality due to specific diseases (2). The GBD 2010, published in 2012, is an extensive and comprehensive report on global burden of disease worldwide. Two years earlier, the Ontario Burden of Infectious Diseases Study (ONBOIDS) was published. A somewhat different approach was used in the population summary measures calculated for the province.
This paper was developed as part of a project conducted by the National Collaborating Centre for Infectious Diseases (NCCID) to increase understanding of the burden of infectious diseases in Canada. It also contributes to a parallel project at the NCCID that focuses on the burden of influenza and influenza-like illnesses. Part 1 provides an overview of the considerations used in calculating burden of disease, looking at the strengths and limitations of various approaches. Part 2 provides a summary of key papers analyzing and discussing methods used in the GBD 2010 in greater detail, as well as the approaches used and the results from the ONBOIDS.
Part 1: Calculations used in Burden of Disease Measurement
While individuals generally know when they are healthy or sick, there is no consensus about how to define the health of a population or on how much a given population is affected by illness or disease. For many years, population health was evaluated using mortality-based indicators only. In other words, the health of a population was determined by how many people died and why – the causes and rates of death. Although mortality-based indicators are useful, they do not provide all the information necessary to assess the health of a population or to compare the effectiveness of interventions to protect or improve health (5). That is, they do not take into consideration the effects of being ill, perhaps for many years, before death or recovery. Summary measures provide a fuller account of the health of a population because they include estimates of the effects of morbidity as well as mortality.
Health-Adjusted Life Years (HALYs) are summary measures of population health used in burden of disease estimates. They combine the effects of disability or disease (morbidity) and death (mortality) simultaneously. HALYs, an umbrella term for a number of such summary measures, allow for comparisons to be made across illnesses, interventions, and populations (5). The data are normally presented by age, sex, and region.
To calculate the HALYs of a disease, three general steps are required. As Gold et al., (5) describe, researchers must:
- Describe the associated state of health (“health state”) or disease conditions;
- Develop numerical values or weights for the health state or condition;
- Combine the numerical values of each health state with estimates of life expectancy.
Each of these steps includes choices about methods and social value, which will be described below, that affect the final estimates. The morbidity components of HALYs are referred to as Health-Related Quality of Life (HRQL) and are represented on a scale of 0 to 1. Two common measures of HALYs, Quality-Adjusted Life Years (QALYs) and Disability-Adjusted Life Years (DALYs), are described in detail below. As will be seen, QALY and DALY have different purposes and use different approaches to calculate HRQL associated with disease conditions or good health (5).
1.1 Estimating Quality-Adjusted Life Years (QALYs)
QALYs represent statistical values ascribed to health outcomes, both in terms of quantity and quality. Developed in the late 1960s by economists, QALYs are mainly used for cost-effectiveness analyses of clinical interventions and improvements in social welfare (5). The QALY method can estimate the number of years lived and the quality of life during those years that can be attributed to an intervention. When combined with the cost of providing an intervention, QALYs are used to develop cost-utility ratios required to generate a year of “perfect health”, a perception of life without pain or disease (5). In a context of limited resources, QALYs measure the “utility” of an intervention – its potential effect on quantity and quality of life – and are used to determine where resources should be allocated (5, 6).
About QALYs
Quality-adjusted life years are usually used to analyse clinical interventions.
The goal is to maximize the “good” of quality of life.
QALYs use utility weights (0 = death and 1 = perfect health) generated through techniques such as standard gamble (asking respondents to assess which health states are more valuable to them).
In QALY estimates, the HRQL (or utility values) are not linked to specific diseases but rather are based on individuals’ opinions about their own health state (patient weights) or on the judgements of others (e.g., a representative sample of the population, study researchers, or health professionals) about a particular health state (community weights) (5). HRQL is typically generated through time trade-offs or standard gamble techniques. In other words, respondents are asked to generate health state values by making clear what they would be willing to sacrifice in order to return from a defined state of poor health to perfect health, where a year of perfect health is worth a value of 1 and death is considered to be 0. If the year is not spent in perfect health (e.g., the individual has to live with chronic pain), the value assigned is between 1 and 0. This score takes into account five quality of life dimensions: a) mobility, b) pain or discomfort, c) self-care, d) anxiety-depression, and e) usual activities (6). QALY estimates therefore integrate bio-medical and psycho-social aspects of the burden of a disease.
QALYs can measure both the effectiveness and the cost-effectiveness of an intervention. For example, QALYs can compare an intervention that can help prolong life but has serious side effects (such as permanent disability caused by radiation or chemotherapy for cancer), with an intervention that improves quality of life without prolonging it (such a palliative pain management). The measure can give an idea of how many extra months or years of life of reasonable quality of health a person might gain with each intervention (7). QALYs are calculated by multiplying the number of years of life added, by the HRQL. (6).
QALYs = additional number years of life x HRQL
1.2 Estimating Disability-Adjusted Life Years (DALYs)
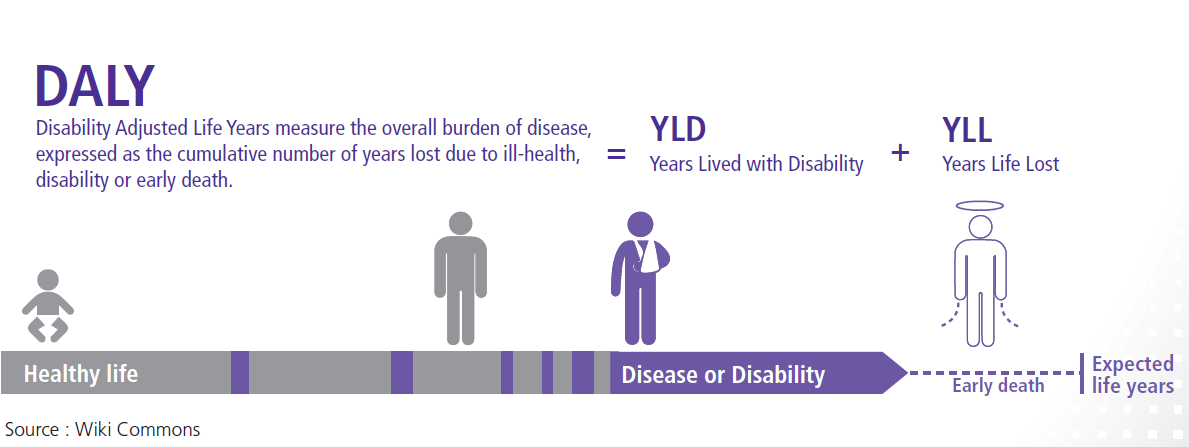
The DALY method was developed in 1990 by researchers at the World Bank and Harvard University to quantify the burden of disease and disability in populations. It measures the difference or gap between the current health of a population and an ideal situation; i.e. where everyone reaches the standard life expectancy in perfect 4 The DALY method is based on an assumption that time is the most appropriate measure for burden of disease, including the time lived with disability and the time lost due to premature mortality(8):
DALY= Years of life lost due to premature mortality (YLL) + Years lived with disability (YLD)
The YLL measure corresponds to the number of deaths multiplied by a standard life expectancy.
YLL = N x L
Where:
N = number of deaths
L = standard of life expectancy at age of death in years
The YLD is derived by multiplying the number of disability cases (incidence cases) by the average duration of the disease/disability and weighted representations of disability called the disability weight (DW) (8).
YLD= I x DW x L
Where:
I = number of incident cases
DW = disability weight
L = average duration of the case until remission or death (years) (9)
The GBD 2010 study used a YLD calculation based on prevalence rather than incidence of disability (9):
YLD = P x DW
Where:
P = number of prevalent cases
DW = disability weight
About DALYs
Disability-adjusted life years are an absolute measure used to compare disease burden in populations.
The goal is to minimize the “bad” of gaps in health.
DALYs use disability weights (0=perfect health and 1=death) which are generated through consultations with clinicians, experts or community.
Disability weights are used to represent the HRQL in DALYs based on non-fatal health outcomes as described in the International Statistical Classification of Diseases, Injuries and Causes of Death (ICD) (10). Once a condition/ disability or illness is described, its non-desirability is assigned a value on a scale from 0 and 1, where 0 represents perfect health and 1 represents death. (Note that this is the reverse of the scale used for QALYs.) The HRQL weights reflect the preferences that people or society have for each health state. As with QALYs, HRQL weights for DALYs are determined by asking respondents to assign disability weights to a specific disease or health condition by using one of several trade-off techniques (5).
In addition to disability weights, DALY estimates have also historically included a weighting for age. Typically, this approach favours young adults who are “productive” in their communities and contributing to the economy, leaving out the very young and older adults, who are more dependent. The GBD 2010 no longer included age weights, based on critiques that this method devalued the lives of the “non-productive” members of society.
HRQL in QALYs and DALYs
HRQLs used in QALYs and DALYs are inverted: QALYs measure equivalent healthy years lived, whereas DALYs measure loss of health years.
In QALYs HRQL weights are based on the values placed on a state of health.
Disability weights and age weights are not the only social values that are factored into DALY estimates. Murray and Lopez (4) identified other considerations that influence how and why DALYs and other HALY estimates are made, including:
- How long “should” people live?
- Is a year of healthy life gained now worth more to society than a year gained sometime in the future? (This time trade-off is called discounting)
- Are lost years of healthy life valued more at some ages than at others? (age weighting)
- Are all people equal? Do all people of the same age lose the same amount of health when they die? (4,8)
Values ascribed to these social questions are controversial, as will be discussed later, and as a result, not all DALY estimates include disability weights (see section 1.4.2). Despite their limitations, DALY estimates are currently the most common methodology used to determine HALYs in burden of disease estimates as they are considered the best indicators available now to set priorities for resource allocation (9).
1.3 Data Required to Calculate Burden of Disease
HALYs, including DALYs and QALYs, are based on the latest available epidemiological data.
To measure HALYs the following information is required:
- Mortality (number of deaths)
- Disease incidence
- Health state distribution
- Health state duration
- Weights associated with each health state.
The information needed can be retrieved from different sources: vital statistics, data from reportable diseases, healthcare utilization data, country censuses, national and local surveillance data, verbal autopsies (in countries where they are used), hospital records, surveys (e.g. road safety surveys, institutional survey, household survey, health survey, etc.), police records, death certificates and mortuary records. (It is important to note that health conditions are often under-reported or under-diagnosed.) In an ideal world, all the data would be valid, timely, locally-derived, and disaggregated by age and sex (10).
Calculating burden of disease can be a challenge as appropriate sources of data must be available. For this reason, calculating burden of disease for developing countries may be particularly problematic. One of the biggest challenges is to ensure that data from different sources and different countries are of the same quality. To facilitate this process, Lozano et al., used a six-step method, briefly described in the text box below, to assess and enhance data quality for completeness and diagnostic accuracy, to deal with missing data and stochastic variations, and to extrapolate the probable cause of death (10). For more detail on the complete method, refer to Lozano et al. (10).
CALCULATING THE GLOBAL BURDEN OF DISEASE
Lozano et al. describe six steps used to calculate burden of disease in the GBD 2010:
Step 1: Assessment of completeness of death recording in each source
Because death records in some countries are incomplete, particularly for children under the age of 5, a number of statistical models were used to assess the completeness of the death records.
Step 2: Mapping revisions and variants of the ICD
Vital registration data from 1980-2010 are based on different ICD reports. Data were mapped to show these versions and any revisions to the ICD 9, ICD10 as well as the basic ICD 9 (BTL).
Step 3: Redistribution of deaths assigned to garbage codes
Garbage codes represent official causes of death in data sets that should not have been identified as such. This step reassigns poorly coded deaths to probable underlying causes.
Step 4: Age splitting and age-sex splitting
Different data sources use differing age groupings. To be consistent, the data in the 2010 GBD were assigned to standard age groups (i.e. 0-4; 5-14; 15-44; 45-59; 60+). In cases where both sexes were combined in death reports, data were re-assigned to age-sex groups.
Step 5: Data smoothing
In some countries, very few deaths (0-2) might be reported for a specific cause in some years due to stochastic variations. This step uses a standardized smoothing algorithm to decrease the bias introduced by those deaths in the calculation of GBD.
Step 6: Outlier description
Despite all the efforts to ensure data quality and comparability, some data might still seem completely implausible. When there is a large inconsistency for a given disease with other data source from the same country at the same time, these data are considered outliers and are excluded from the analysis.
Source: Lozano et al. (10)
1.4 Limitations, Advantages, and Disadvantages of DALYs and QALYs
Calculating the burden of disease using QALYs and DALYs gives important information for policy-makers. However, the aspects of health that are valued as well as the populations from whom values are gathered differ between QALYs and DALYs. Life expectancy is also handled differently in these two approaches. It is important to understand the limitations, advantages, and disadvantages of these two methods to make appropriate decisions when calculating burden of disease.
1.4.1 QALYs
While QALYs provide an indication of the benefits gained from a medical intervention in terms of quality of life, their usefulness is debated. In particular, critics have asked, “Is it really possible to define perfect health?” Further, it has been suggested that some health states might be deemed worse than death, perceptions which also vary depending on culture and other aspects of society. QALYs have also been found to lack sensitivity when comparing two competitive but similar drugs or when evaluating treatments for less severe health problems (6).
The effects of chronic diseases, where quality of life is compromised but survival is not, are also difficult to address using QALYs. As a result, disease-specific measures of life quality such as DALYs may be more appropriately used. Likewise, because QALYs rely on dimensions of age, context, and responsibilities in different life stages, they can be difficult to quantify if a preventative measure will not have an effect on health outcome in the short-term. Another criticism of QALYs is the lack of weight associated with emotional or mental health, and the impact of these problems on quality of life for individuals and their families (6).
Despite these limitations, QALYs are among the few methods that allow for comparisons between interventions or across disease areas. Using QALYs for decisions on resource allocations helps to articulate the choices to be made between competing medical therapies or technologies (6). However, Malek has cautioned that QALYs should not be used in isolation to inform decision-making because they do not capture all the domains and aspects of healthcare (11).
1.4.2. DALYs
In DALYs, the HRQLs are associated with a specific diseases, rather than with a state of health. This is done to avoid self-assessment of health, which could potentially bias the estimates, especially for purposes of cross-cultural comparison. Indeed, this approach helps to get a better estimate in a population that may have a high mortality rate but has a tendency to not describe their health as poor for cultural reasons (5). But DALY estimates do not capture other aspects of a disease, such as psychological effects on relatives (12).
Age weighting is probably the most controversial societal value to be built into the DALY estimates. When including age weights as a criterion for priority setting, a year of healthy life at younger and older ages is given less weight than a year of health at other ages; that is, a year of life in young adulthood is valued more than a year life in childhood or old age. (4). In many societies, this reflects an interest in productivity and receiving a return on investment in the education of children. In fact, one of the main concerns about DALY is that it is mostly an economic measure of productive capacity for the affected individuals. Some researchers and information users do not agree on this principle or on the magnitude of the difference between the ages, and so choose to omit age weighting (8). Because of disagreements about the merits of age weighting, neither the GBD 2010 study nor the ONBOIDS included age weights.
Using discounting – comparing the value of a healthy year lived now against the value of a healthy year in the future – has also been criticized as a social value choice because of the subjectivity of this criterion. Different cultures or countries might value current years of health differently from healthy years in the future. In the GBD 2010 report, estimates for future years were generally discounted at a rate of 3%. This means that a year saved next year is worth 97% of a year of life saved this year (9). When comparing burden of disease estimates, it is important to know which social values have been included in the reports as results will be different according to the weights that were included.
1.4.3. Ethical issues related to QALYs and DALYs
Gold et al. (5) mentioned three major ethical issues associated with QALY and DALY estimates:
- QALYs and DALYs discriminate against those members of society who are already at a disadvantage socially or in relation to their health. Based on actual calculations, older persons or people with pre-existing disabilities are considered a bad investment as there is limited potential for health improvement, which leads in turn to a lower HALY value;
- Both methods also discriminate against people with limited treatment options and/or recovery potential – a related but slightly different concern to the first one above;
- QALYs and DALYs also fail to account for qualitative differences in health outcomes. Because the values for health states/diseases are combined across individuals, and across the spectrum of being alive to death, there is no real distinction between life-saving interventions and health-improving interventions. This aggregation also raises the question of whether minor benefits accruing to many individuals should be valued to the same degree as significant benefits accruing to a few individuals (5).
New techniques that better reflect social values are in development and might help not only to solve some of these ethical problems, but also to get a better view of population health. Gold et al. note that cost-effectiveness studies should be seen as only one of several inputs for decision-making. Consideration must be given to how need is distributed in a population, how to set priorities for illnesses that are rare or expensive, and how to balance health status improvement versus saving lives, as these values can distort HALY estimates.
Despite the different disadvantages associated with DALYs and QALYs, they each provide important information about the burden of diseases. The 2010 GBD and the ONBOIDS only used versions of DALY estimates, as they were not investigating treatments or interventions. Some summaries from these studies are reviewed in Part 2.
Part 2: Literature Review of the 2010 GBD and the ONBOIDS
2.1 The Global Burden of Disease 2010
The results from the GBD 2010 report were published in several papers in The Lancet in December 2012 (Lancet, volume 380, issue 9859). Healthy life expectancy (HALE), DALYs, YLL, YLD, risk factors, disability weights and age- and sex-specific burden of disease were estimated for the year 2010 and recalculated for 1990. In this section we provide a brief discussion of the methods and findings published, in the order the papers appear in the Lancet issue. The methods and findings of the GBD 2010 can be found in GBD: Generating Evidence, Guiding Policy (13).
2.1.1 Mortality – Lozano et al. 2012
Lozano et al. estimated the number of annual deaths from 235 causes between 1980 and 2010 for the world and for 21 regions, with uncertainty intervals, separately by age and sex. In the data collection, they found that vital registration systems (including medical certification of the cause of death) capture only about 18.8 million of the 51.7 million estimated deaths in 2005 (10).
In 2010 there were 58.2 million deaths worldwide. At the most aggregate level, communicable, maternal, neonatal and nutritional causes represented 24.9% of those deaths, which was down from 34.1% in 1990. Deaths from non-communicable diseases increased proportionately world-wide, but at the same time people around the world were living longer compared with 35 years earlier (the average age of death has increased 35 years since 1970; although in portions of sub-Saharan Africa, the increase has only been about 10 years (13)), with a concurrent shift to older populations in general. Children under the age of 10 and females of all ages have demonstrated the greatest shifts to longer lives.
The authors found that since 1990, age standardised death rates for some key disorders rose (HIV/AIDS, Alzheimer’s disease, diabetes mellitus, and chronic kidney disease), while death rates fell for most diseases. Among the top five causes of death in 2010, chronic kidney disease increased the most (211%), followed by “other” musculoskeletal conditions and diabetes, which grew by 88% and 75% respectively. Among communicable, nutritional, newborn, and maternal conditions, lower respiratory infections and diarrheal diseases showed the greatest declines, with decreases of 65% and 83% respectively.
2.1.2 Disability weights — Salomon et al., 2012a
Salomon et al. re-estimated the disability weights for the GBD 2010 according to the perception of the general population, which was determined “through a large-scale empirical investigation in which judgments about health losses associated with many causes of disease and injury were elicited from the general public in diverse communities through a new, standardised approach” (14).
Two methods were used to elicit opinions of the general population: 13,902 individuals (18 years old or older) from Bangladesh, Indonesia, Peru and Tanzania were interviewed through a household survey and by telephone; and 16,328 answered an open-access web-based survey. Each respondent had to consider two hypothetical individuals with different, randomly selected health states and indicate which person they thought was healthier. Salomon et al. used these responses to develop a disability weight scale from 0 (no loss to health) to 1 (health loss is the equivalent of death).
In contrast to the critique that variability of disability weights in DALY estimates may be highly dependent on particular social or cultural environments, as described in Part 1, the authors of this paper found that disability weights were similar among cultures, which suggests a broadly shared perception both of what creates disability and levels of severity. For example, respondents consistently identified health states such as acute schizophrenia and severe multiple sclerosis as very severe, while ranking mild hearing loss and long-term treated fractures low on the severity scale.
Salomon et al. noted that there are limitations to this new method of estimating disability weight. First, while the household survey included a set of countries that represent different cultures and different levels of economic development, the five household surveys did not represent a random sample of the world’s population; the results might have been different if another set of countries has been chosen. The same was true for the web-based surveys for which North America, Australia, and Western Europe were over-represented compared to other parts of the world. The use of lay language might also have contributed to omission of some aspect of the health state and/or the severity of the health states and could have influenced the disability weight attributed to each health state (14).
2.1.3 Measure of healthy life — Salomon et al., 2012b
Healthy life expectancy (HALE, also frequently known as health-adjusted life expectancy) was measured by Salomon et al. (15). HALE is related to HALYs, but is not disease-specific (5). HALE represents the number of years that a person at a given age can expect to live in good health. It summarizes mortality and non-fatal outcomes in a single measure of average population health, and has been used to compare health between countries and to measure changes over time.
In their article, Salomon et al. calculated HALE using the life table method, incorporating estimates of average health over each age interval. The results from the HALE of the GBD 2010 suggest that the world’s population loses more years of life due to disability than it did 20 years ago, and that women lose more years to disability than men. Their findings also showed that global male HALE at birth was 59 years and global female HALE at birth was 63.2 years. HALE has increased more slowly than life expectancy over the past 20 years and HALE differs substantially between countries (15).
2.1.4. Years lived with disability — Vos et al., 2012
The GBD 2010 examined 291 diseases or injuries. According to Vos et al., 289 of these diseases and illnesses cause disability, which represented a total of 1,160 sequelae (16). Data from published studies, case notification, population-based disease registries, antenatal clinic sero-surveillance, hospital discharge data, ambulatory care data, household and other surveys, and cohort studies were gathered to perform this analysis.
YLDs were computed as the prevalence of a sequela multiplied by the disability weight for that sequela without age weighting or discounting. The YLDs of each disease or injury were the sum of the YLDs for each key sequela associated with that disease. Sequelae could include the disease itself (e.g. diabetes) or the outcomes associated with that disease (e.g. diabetic foot, neuropathy or retinopathy). Each sequela was identified and its incidence, prevalence, remission, duration and excess mortality documented. Two estimates of YLD were calculated: the disability weights for individuals with single sequela and the disability weights for individuals with multiple sequelae. Disability weights for 220 unique health states were used to capture the severity of health loss. YLDs were calculated by age, sex, country, and year, and were adjusted for comorbidity with simulation methods. For the first time, uncertainty estimates at all stages of the analysis were included.
The results showed that the main contributors to the global YLDs were mental and behavioural disorders, musculoskeletal disorders, and diabetes. The specific causes of YLDs were similar between 1990 and 2010: low back pain, major depressive disorder, iron-deficiency anemia, neck pain, chronic obstructive pulmonary disease, anxiety disorders, migraine, diabetes, and falls. Population growth and population aging have also increased YLD numbers. The high rate of YLD due to mental and behavioural disorders and musculoskeletal disorders showed that health systems will need to address the issue of an increasing number of individuals with disorders causing disability rather than mortality. This also demonstrated the importance of including YLD in the summary of population health for decision-makers.
The authors noted that one strength of this study was the use of the general population, rather than small panels of healthcare professionals, to determine disability weights, as described above. However, there was still the concern that the brief descriptions of different health states in lay language cannot necessarily properly explain all the implications of the health states described.
Overall, the GBD 2010 analysis of YLDs provided important insights into which types of data are important to gather for assessing non-fatal health outcomes. It also demonstrated that quantification of health loss in populations is feasible by using comparable metrics that identify the leading causes of non-fatal illness in different regions at different ages and different points in time (16).
2.1.5 Assessment of risk factors in the GBD — Lim et al. 2012
The study by Lim et al. is the first analysis that assessed changes in burden of disease attributable to risk factors over time. They estimated the DALYs attributable to the independent effect of 67 factors for 21 regions in 1990 and 2010 (17).
The approach they used was to calculate the proportion of disease burden caused by specific risk factors holding other independent factors unchanged. A three-level hierarchical organization system was established. Level 1 risks were clusters of risk factors linked by mechanism, biology or potential policy intervention. Level 2 risks represented the major proportion of the risk factors themselves. For some risk factors, such as occupational carcinogens, a third level was added to provide additional detail. The burden of disease attributable to 67 risk factors was calculated. Population risk factors associated with physiology and air conditions were excluded due to analytical challenges.
Their analysis showed that the three leading risk factors for GBD were high blood pressure, tobacco smoking (including exposure to second-hand smoke) and household air pollution from solid fuels. Between 1990 and 2010 there was a large observable shift in the risk factors associated with GBD from communicable diseases to non-communicable diseases. However, the global level of analysis of risks to health masks important regional variations. For example, even if non-communicable risk factors were the major disease burden in sub-Saharan African in these years, communicable risk factors continued to cause a disproportional amount of health burden.
Although many of the disease and risk factors were present at the same rate worldwide, there were some discrepancies. For example, diarrhoea and HIV/AIDS were leading cause of DALYs, but their risk factors were under-represented. Unprotected sex was not included as a risk factor in this study as it was almost impossible to get consistent and reliable data source and estimation. The authors noted that this kind of variation could be important for considering future estimates and calculations of risk factors (17).
2.1.6 Measure of DALYs — Murray et al., 2012
Murray et al. calculated DALYs for 291 causes, 20 age groups, and both sexes and for 187 countries (3). YLLs were calculated from age-, sex-, country-, and time-specific mortality by cause. YLDs were calculated as prevalence for 1,160 disabling sequelae, by age and cause, and weighted by new disability weights for each health state. In response to critiques of the 1990 GBD, neither YLLs nor YLDs were age-weighted or discounted. Furthermore, YLLs were calculated with reference to a new standard life expectancy at each age.
The GBD 2010 estimated that the number of DALYs for the world in 2010 had decreased by 0.5% from the estimates for 1990. Even if relatively small changes in the number of DALYs have occurred, Murray et al. observed an important shift in DALY composition. Indeed, contribution of deaths and disability among children declined from 41% of global DALYs in 1990 to 25% in 2010. In 1990, 47% of DALYs worldwide were from communicable, maternal, neonatal, and nutritional disorders, 43% from non-communicable diseases, and 10% from injuries.
By 2010, these figures had shifted to 35%, 54%, and 11% respectively. Thus, global disease burden had shifted away from communicable to non-communicable diseases and from premature death to years lived with disability. Differences across regions also highlighted the importance of understanding local burden of diseases (3).
Even if the availability and quality of the data were a major limitation of this study, the GBD 2010 represented a great increase in scope from the preceding GBDs and a good revision of the GBD 1990. More risk factors, a larger disease and injury list, and more age groups were included. Many of the key limitations from previous studies were also addressed, including eliminating discounting or age-weighted or taking co-morbidity into account for the YLDs estimates (3).
2.2 Ontario Burden of Infectious Disease Study (ONBOIDS)
The ONBOIDS was published in 2010 (18).
To measure the burden of infectious diseases, the authors of this study used a HALY that they considered neither a DALY nor QALY as a health gap measure to assess both premature mortality and the reduced functioning associated with diseases or injuries. As they did not use disability weights and did not discount age, as had been done in previous GBD studies, the ONBOIDS authors did not adopt the term DALY. (This pre-dates the release of the GBD 2010 papers, which also did not use age weighting, as noted above.) In the ONBOIDS:
HALY =Years of life lost (YLL) + Year-equivalent of reduced functioning (YERF)
YERFc,h,a,s = Ic,h,a,s x Dc,h x SWc,h
Where:
Ic,h,a,s = incident cases by cause (c), health state (h), age (a) and sex (s)
Dc,h = average duration of health state
SWc,h = severity weight associated with health state
Severity weight was taken from the Classification and Measurement Systems of Functional Health (CLAMES) previously adopted by Statistics Canada and in use at that time by programs in the Public Health Agency of Canada (18).
The disease burden was estimated by pathogen (e.g. Streptococcus pneumonia) and by syndrome (e.g. pneumonia). ONBOIDS took into consideration 51 pathogens and 16 syndromes that were severe enough to require health care or that were reportable. It is important to note that the authors adjusted the data for under-diagnosis and under-reporting.
To reduce year-to year variation, the authors estimated annual disease incidence and mortality by calculating an average over three years from the latest data available. Life expectancy was extracted from the Census of Canada and Ontario life expectancies for women and men were used as the standards.
The study found that each year in Ontario, there are over 7 million episodes of infectious diseases, which result in nearly 4,900 deaths. Infectious diseases accounted for 82,881 HALYs, comprising 68,213 years of life lost due to premature death and 14,668 year-equivalents of reduced functioning. The overall burden of infectious disease represents roughly 25% of burden of all cancers. The burden of infectious diseases was similar for males and for females, although there were sex specific differences.
The ten infectious agents contributing the most to burden of disease were: Hepatitis C virus, Streptococcus pneumonia, human papilloma virus, hepatitis B virus, Escherichia coli, HIV/AIDS, Staphylococcus aureus, influenza, Clostridium difficile and rhinovirus. The five most burdensome syndromes were pneumonia, septicaemia, urinary tract infections, acute bronchitis and endocarditis. Women had greater HALYs for HPV and urinary tract pathogens while HIV, HBV and HCV showed a greater predominance among men.
The results of the study also showed that a large proportion of the burden of disease could be attributed to a small number of pathogens and syndromes for which there are highly effective targeted interventions (ex: pneumonia or HPV vaccine) and non-specific interventions (e.g. condoms, hand-washing). This means that the future burden of some of these pathogens/syndromes could be dramatically reduced with greater uptake of available interventions. A significant proportion of disease burden occurred in health care settings (e.g. E. coli, S. aureus), which suggests the need for specific interventions to minimize transmission of these pathogens.
The use of a pathogen-based approach, the inclusion of a broad range of health states, and the use of linkable health care utilization data are among the more important strengths of this study (18). Similar to the majority of studies analyzing GBD, suboptimal quality and availability of data, study scope not extended to economic or psychosocial impacts, etiologic agent distributions, the exclusion of certain important infectious agents, syndromes or health states, and the burden of undiagnosed and unreported cases are the main weaknesses the authors identified for this study (18).
Summary
This paper has provided a brief summary of the main approaches to calculating burden of disease as well as summaries of recent work from the Global Burden of Diseases study and a 2010 report from Ontario.
It is important to understand what burden of disease studies measure, including the assumptions that are made in developing the basic formulas for HALYs. Data sources and methodologies used as well as decisions about weights given to different states of disability and poor health, all need to be taken into consideration when assessing results. As many authors have pointed out, seemingly objective measurements of disease burden still reflect the social values of the societies and researchers who undertake them.
It is also important to mention that reports on broad global patterns may mask enormous regional variation in burden of disease. For example, in Sub-Saharan Africa, childhood underweight and household air pollution due to solid fuel are the major risk factors in the burden of disease while, according to the latest GBD, global estimates showed a significant decrease in the importance of those two risk factors (17). It is important to take this issue into consideration variation between provinces, rural versus urban settings, or northern versus southern populations in Canadian estimates of burden. Having a clear understanding of the regional distribution and variation of the risk factors across Canada will be important to estimates of DALYs, QALYs and HALYs.
References
(1) Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis 2007 Nov 7;1(2):e114.
(2) About the global burden of disease. 2013; Available at: http:// www.who.int/healthinfo/global_burden_disease/about/en/index. html.
(3) Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012 Dec 15;380(9859):2197-2223.
(4) Murray CJL, Lopez AD, Harvard School of Public Health, World Health Organization, World Bank. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Cambridge, MA: Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank ; Distributed by Harvard University Press; 1996.
(5) Gold MR, Stevenson D, Fryback DG. HALYS and QALYS and DALYS, Oh My: similarities and differences in summary measures of population Health. Annu Rev Public Health 2002;23:115-134.
(6) Phillips C. What is a QALY? 2009.
(7) Neumann PJ, Zinner DE, Wright JC. Are methods for estimating QALYs in cost-effectiveness analyses improving? Med Decis Making 1997 Oct-Dec;17(4):402-408.
(8) Young TK. Population health: concepts and methods. 2nd ed. New York, N.Y.: Oxford University Press; 2005.
(9) Metrics: Disability-adjusted life year (DALY). 2013; Available at: http://www.who.int/healthinfo/global_burden_disease/metrics_ daly/en/index.html.
(10) Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012 Dec 15;380(9859):2095-2128.
(11) Malek M. Implementing QALYs. 2001.
(12) Thacker SB, Stroup DF, Carande-Kulis V, Marks JS, Roy K, Gerberding JL. Measuring the public’s health. Public Health Rep 2006 Jan-Feb;121(1):14-22.
(13) Institute for Health Metrics and Evaluation. Global burden of disease: Generating evidence, guiding policy. Seattle, WA: IHME; 2013.
(14) Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012 Dec 15;380(9859):2129-2143.
(15) Salomon JA, Wang H, Freeman MK, Vos T, Flaxman AD, Lopez AD, et al. Healthy life expectancy for 187 countries, 1990-2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet 2012 Dec 15;380(9859):2144-2162.
(16) Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012 Dec 15;380(9859):2163-2196.
(17) Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012 Dec 15;380(9859):2224-2260.
(18) Kwong J, Institute for Clinical Evaluative Sciences in Ontario. Ontario burden of infectious disease study. 2010.
Production of this document has been made possible through a financial contribution from the Public Health Agency of Canada through funding for the National Collaborating Centres for Public Health (NCCPH).
The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada. Information contained in the document may be cited provided that the source is mentioned.
