Introduction
Public health practitioners, whether they are employed in front-line care or policy and planning, are decision-makers. They are trained and skilled in making decisions to prevent and control disease, as well as the conditions that lead to disease and death. In doing so, public health professionals and the governmental bodies they work with must determine priorities for health research and investment in health interventions (Lajoie, 2015). An important step in making these decisions is to understand the “burden” of a disease; that is, the “duty, obligation, and expense” (as defined in the Oxford English Dictionary, 1995) that the disease creates for society, as well as the individuals concerned. Burden of disease may be seen as the cornerstone of population health decision-making, used by public health policy makers at all levels to describe and to try to understand population-level health issues and prioritize among remediable, burden-producing problems.
Indeed, the term ‘burden of disease’ is ubiquitous in the population and public health literature, although so rarely defined that it might pass as un-noteworthy and unimportant. That the term is often preceded by a definite article, that is, ‘the’ burden of disease, suggests there is clarity, consensus, and uniformity in what the term means and how it is used. Yet it is not uncommon to find population and public health text books that do not include burden of disease among the basic concepts named and defined, despite frequent mentions of the Global Burden of Disease project and definition of Disability Adjusted Life Years (DALYs), the summary measure of burden espoused by that project (Donovan et al., n.d.; Starfield, 2001; Young, 2004). Although the GBD approach is well articulated in population health literature, it is less clear what authors mean by burden when they are not referring to the GBD body of work. Moreover, researchers in a variety of disciplines have argued that a biomedical model and quantitative measurement are not sufficient to understand true burden, and that aggregate and averaged measures of burden can mask critical differences within populations, especially where there are inequities that make some sectors of a population particularly vulnerable to disease.
Recognizing these information needs, challenges, and contexts, the National Collaborating Centre for Infectious Diseases (NCCID) has prepared this concept paper as a starting point for a critical examination of burden of disease, with the longer-term goal of developing a framework to guide transparent, fair, meaningful, and practical measures of burden. Consideration of the concept of burden of disease, in general, is combined with reflection on special considerations or implications for the burden of infectious diseases. The objective of this paper is to build broader awareness for potential ‘burdens’ and to initiate conversations among experts in population health measurement, theorists exploring causal pathways for infectious disease, medical ethicists, and front-line public health practitioners. The initial task is to plumb and map the conceptual construct of burden of disease, to gain understanding of the depth and breadth of meaning that has accrued to the term, as well as explore examples of current applications in research and policy circles. This will allow us to lay out a series of omissions and unaddressed questions that could be incorporated in a broader framework of burden than has been traditionally applied. NCCID invites our readers to join in a discussion of this foundational work, particularly as it applies to infectious disease public health. We welcome your comments and remarks.
Which Burden, Whose Burden is ‘The’ Burden of Disease?
Given its importance for guiding public health decision-makers, the determination of burden cannot be based merely on expediency of what can be easily measured or determined by the loudest voice among competing health advocates. Efforts to ground disease burden in evidence and lend consistency and rigour to its assessment have led to numerous summary measures. Summary measures of population health are widely used because they provide understandable representations of complex epidemiology that can help expedite the development of disease prevention and control strategies. Murray and colleagues describe a range of objectives that summary measures of burden of disease serve. They may be used to:
- monitor change in a population’s health
- compare the health of one population with that of another
- identify and quantify overall health inequalities within populations
- balance attention to effects of non-fatal health outcomes
- provide data for cost-effectiveness analysis
- improve curricula
- inform priorities for research investment
- inform priorities for health services with information on particular diseases and on risk factor contributions (Murray, et al., 2000).
Common themes among these objectives are needs for information to draw fair comparisons, prioritize actions, use resources wisely, and to test theories of causality. In a broad sense, measures of burden support better decision-making and focus actions taken by public health. However, it is necessary to understand the basic elements of burden measures and the essential information needs of public health decision-makers.
To understand whether the needs of public health are being met by burden of disease measures, we may begin with the question: what basic information might we expect such measures to provide? Presumably, burden of disease should provide information on both the frequency and severity of health conditions to help assess their consequences alongside other competing priorities. Information on frequency alone is understood to be insufficient, since there are many illnesses, such as the common cold, that are mild and self-limiting. However, we might question whether the causal or mediating effects of mild infectious diseases on other conditions are understood well enough to discount their burden altogether. Also, there may be uncertainty about whether clinical judgement is sufficient to determine which illnesses pose inconsequential effects without also accounting for the real and often complex experiences of those affected, such as men and women who have recurrent, though mild, infections. Likewise, knowledge of illness severity alone is insufficient; public health officials temper information on disease severity with information about how often a diagnosis is made in the population. Yet questions may arise about whether this occurs in practice when it appears that public health priorities are influenced by concerns over perceived severity, or by the frequency and severity of disease in other countries—some of which differ markedly from Canada for both determinants of health and public health resources that influence disease outcomes.
Burden of disease measures should also direct our attention and resources to those conditions that produce a large absolute or relative impact on population health—they tell us about non-normative and unprecedented outcomes, or whether a problem is getting worse or better. Whether public health professionals can identify what threshold should be surpassed before an absolute burden can be recognized, or what amount of change signals worsening burden are added methodological challenges with practical implications for measuring disease burden.
We might also expect burden of disease measures to be meaningful and sensitive in reflecting societal values for which health conditions and consequences constitute an unacceptable or unfair problem. Burden of disease measures should be practical and feasible or discernable in terms of the real world context of public health practitioners and how they might observe, know about, and measure health consequences. Above all else, we could expect burden of disease measures to be explicit and transparent so that all can understand the basis for public health policy decisions.
In current public health contexts, clear understanding of challenges to public health and a framework to guide burden of disease assessment is more important than ever. In a time of fiscal constraint, it becomes all the more important to estimate burden of disease and rely on this evidence to take actions that result in the greatest gains possible. As well, because public health operates within a multi-disciplinary population health context, and each discipline in this ‘confederacy’ brings its own epistemological and etiological precepts and theories, ‘burden’ has taken on expanded boundaries, which may be rewarding, or altogether confounding. Which burden are we talking about and are we sure we’re talking about the same burden? The ability to answer that question may depend on our ability to collaborate effectively and to build a conceptual framework to guide action on common goals for improving the health of Canadians. Finally, public health professionals work among populations and communities set in conditions that are increasingly recognized as inequitable, making it equally important to be clear on whose burden we are talking about, and explicit about our potential bias as relatively privileged public health professionals. In stating that burden of disease supports better decision-making, we might call public health to a more stringent and specific goal, that is, to plan measures of burden that can reveal inequities that underlie poor health outcomes and support decisions that lead to greater health equity.
Common Usage of ‘Burden’ in Population Health Research Literature
The term ‘burden’ appears in an enormous number of studies that quantify population-level health outcomes. An exploratory search of articles in academic journals for titles containing ‘burden’ found recent research literature to be replete with a variety of related terms and phrases. Although it is beyond the scope of this high-level review to define all these terms, it is interesting to briefly consider the general breadth and depth or precision of terms in common usage, some gross distinctions in meaning that are apparent, and the application of similar and sometimes conflated terms in the context of various disciplines and subject areas.
While burden of disease was the most frequently used variant that appeared in a search of ‘burdens’, in some instances, this term appears to be used interchangeably with Global Burden of Disease (GBD), a well-known project led by the World Bank and the World Health Organization (World Health Organization, n.d.). Burden is a frequently used short-form, and disease burden appears equally common. Burden of illness is a term used within sociological and anthropological domains, and is more inclusive of social and subjective experience. Cost of disease most often signifies an economic analysis, though cost also appears to be used in a more generic sense where the meaning may be synonymous with burden. Physical, economic, and social are commonly employed adjectives that suggest three distinct domains of burden are recognized. Social burden appears to be more obscure in the literature, and some social scientists (Jones & Williams, 2004) have raised concern about the need for a clear definition, limited knowledge of the term among public health professionals, and misuse by some economists who equate its meaning with societal-level economic costs of disease.
Biomedical burden is associated with the use of mortality and morbidity-based measures of population health, though this term may be used more often by those outside biological and medical disciplines than by those within, particularly in criticism of overly narrow population health assessment (Ariana, 2012; Jones & Williams, 2004). Double burden of disease makes references to a theory that developing nations experience a delayed epidemiological transition characterized by persistently high rates of infectious diseases—which may stem from a lack of effective public health measures—as well as escalating rates of chronic non-communicable diseases, attributed either to the adoption of lifestyles ‘imported’ from developed nations (Entsua-Mensah, Doku, & Adzamli, 2012), or poor conditions and shortcomings of social systems (Boutayeb, 2006). However, double burden has also been conceptualized as lower-income women’s experience of compounded structural disadvantage on the basis of both gender and social status, the health implications of which were considered through equity analyses (Whitehead, 1992).
Sociological literature makes use of terms like treatment burden, that is, the burden of undergoing treatment, including time taken to learn about, administer, monitor, or obtain treatment (May et al., 2014; Sav et al., 2013). As well, medication burden—including adverse events, side-effects, drug interactions, and stigma of taking medication—is a term that appears in some sociology of medicine publications (Sav, McMillan, Kendall, Whitty, & King, 2012). A substantive area in sociological literature is research on caregiving burden or carer burden, which has generally explored time devoted to care, economic contributions of caregivers, and self-reported positive and negative effects of care (Barry, 2014; Rivera-Navarro, Morales-González, & Benito-León, 2003; Zan & Scharff, 2014). Particular instruments have been developed to measure caregiving burden, although opinions vary on the best approach (Vella & Pai, 2013). As well, family burden is a distinct area of inquiry, where a separate instrument has been developed to measure burden (Family Burden Assessment Scale) (Maji, Sood, Sagar, & Khandelwal, 2012).
Economic analysts are responsible for a proliferation of ‘burden’ and ‘cost’ terms. Direct, indirect and intangible costs are commonly accounted for. Direct costs are normally associated with hospital care, out-patient treatment, medications (typically, limited to prescribed medications), and visits to physicians (Kwong, et al., 2010). Indirect costs account for absenteeism or lost production (i.e. production in the paid labour force) as a consequence of illness or death, as well as “presenteeism” or reduced productivity at work due to health problems (Malinowsk & Kawalec, 2015). Intangible costs refer to the costs of pain and suffering and to other hard-to-measure kinds of economic costs, including costs to unpaid caregivers and costs associated with undiagnosed disease (American Diabetes Association, 2013), although the scope of what is or is not included does not appear clear. Literature on the burden of mental health disorders and chronic diseases also refers to intangible burden, as well as to subjective burden and personal or individual burden (Englisch et al., 2010; Kobeissi et al., 2011; Zink & Huscher, 2004), which in some instances appears to denote psychological stress or anxiety. Subjective burden refers to self-reports of disease severity, functioning, and quality of life by an affected individual or by their family member, which is distinguished from reports by an objective party, such as an interviewer (Maji, et al., 2012). Economic burdens are also described in relation to the level at which they occur, that is, at individual, household, or societal levels (Fogarty, et al., 2014). A particular focus of household-level burden is the effect of disease or injury on low-income households (Adhikari & Sharma, 2009). Healthcare costs dominate economic analyses of burden, but healthcare burden is also accounted for as part of resource burden, presumably a larger set of resources required to respond to disease. As well, surge volume represents a more specific and short-term healthcare burden (Sills et al., 2011). Finally, healthcare associated burden refers to disease contracted in a health care setting, typically in long-term care or acute care facilities (Taylor et al., 2014).
Attributable burden and risk burden are referred to in studies which associate disease with causal or correlated risk factors, such as the work of Lim et al. (Lim et al., 2012). These studies bring evidence of causal relationships to bear in quantifying what proportion of disease burden can be linked to which proximal (e.g. smoking, activity levels, obesity) or distal risk factors (e.g. low income). However, proximal and behaviour-based risk factors appear more common in the epidemiological literature compared to structural or systemic factors (Link & Phelan, 1995). Among some notable exceptions, Muennig et al. (Muennig et al., 2005) estimated the total burden of disease (measured by LE, HALE, YLL, and HALY) associated with income in the US, and a study from the Netherlands by Cuijpers et al. explored the contribution of childhood adversities, particularly abuse and neglect, to burden of disease (Cuijpers et al., 2011). Some literature also considers co-morbid factors, referring to this as co-morbidity burden or multi-disease burden (Aubert et al., 2015; Talman, 2010). Inequitable burden is a term that appears in some literature that quantifies specific health conditions that disproportionately affect disadvantaged sub-populations (Lennon et al., 2012). However, an overall framework for burden of disease, inclusive of equity concepts and measures, appears to be lacking in the literature.
Milestones in Understanding and Quantifying Burden of Disease
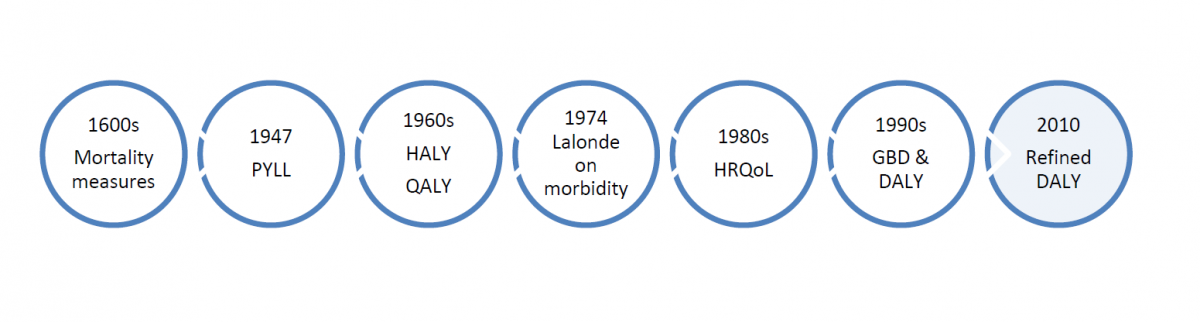
Within the sea of terms used to describe burden of disease, some key ideas and approaches have developed and marked progress in anchoring concepts to practical considerations of measurement and the needs of public health professionals. Over time, the meaning, implicit values, measures, and emphases in burden of disease assessments have undergone changes in response to several influences, including epidemiological trends, improvements in data collection and methods for estimation, and the disciplines and theories that have dominated discourse. It is important to understand some of the key ideas that have been raised in this ongoing conversation on burden of disease (see Figure 1). Some examples follow, though NCCID will engage stakeholders in proposing additions to this basic groundwork.
Mortality rates and proportions
The use of mortality statistics has a long history, dating back to the ‘Bills of Mortality’ in seventeenth century London, where John Graunt analyzed death records and published his observations. The burial records relied upon for mortality data were deficient but nevertheless allowed Graunt to draw important conclusions about certain causes of death, such as plague and murders and, indirectly, about demographic trends and social conditions of that time (Young, 2004). Early analyses of mortality data derived information from structuring the data set by age group and comparing the proportionate contribution of deaths for certain causes relative to the total number of deaths in the population. Several mortality-based indicators, including life expectancy, all cause and disease-specific mortality, and infant mortality are still commonly employed to evaluate population health (Gold et al., 2002). More recently, population health scientists have come to understand that an over-reliance on mortality data may bias judgements of which health conditions are understood to be priorities. That is, some conditions are over-represented in mortality measures because they are more likely to be identified as a cause of death than other co-morbid conditions that contribute to death (Navarro, 2009). Despite public perception that influenza is not a serious health condition, it contributes to mortality from numerous other causes. Szucs cites research from the Netherlands that estimated 1.3 deaths per 10,000 total population were attributed to influenza each year, yet an additional 2.6 deaths per 10,000 population were not attributed to the disease although they were linked to initial influenza infection, reflecting the difficulty of recognizing deaths from complications of influenza (Szucs, 1999). Therefore, mortality-based burden measures are only as accurate as the determination of cause of death allows, and do not account for all important underlying causes of death.
Person years and potential years of life lost (PYLL)
Added refinement to mortality-based measures of burden of disease is attributed to Dempsey (Dempsey, 1947), who in 1947 introduced ‘person years of life lost’, the first of several related concepts and methods that weighted mortality data according to the age-at-death and loss of potential life (Ahrens & Pigeot, 2007). Dempsey’s innovation sought to address the inadequacy of standard mortality-based approaches for measuring the burden of tuberculosis, which affected many young people at that time (CDC, 1986). While Dempsey’s approach centred on life expectancy at birth, others later applied life table estimates (i.e. cohort or period life table) of life expectancy to a given age-at-death, followed by a simplified method that used a standard life expectancy for ease of comparisons between populations with significant differences in life expectancy (Ahrens & Pigeot, 2007). The potential years of life lost measure sums the number of years of life lost by individuals in a population between their ages at death and an arbitrary age, such as 65 or 70 years, to which they may otherwise be expected to survive (Young, 2004). The standard life expectancy adopted varies, though age 70 is commonly used, partly because it is difficult to determine cause of death among more elderly individuals (Ahrens & Pigeot, 2007). Although the idea that premature death is more burdensome or costly to society may seem compatible with dominant cultural norms in Canadian society, the ethical implications of giving priority to young life are contentious.
Considering morbidity
Mortality-based measures of burden are insufficient to assess the impact of disease and injury. Accounting for morbidity enriches population health assessment, particularly for conditions that are less likely to cause death. Some surveillance data exist on particular diseases (e.g. cancer registries) and data are collected on reportable communicable diseases (Thacker, 2006; Lalonde 1974). Administrative records (e.g. hospital admissions, visits to physicians, pharmaceutical use) also provide an important source of data on health care resources devoted to treatment of particular health conditions. Yet data on morbidity remain limited. Morbidity data are also problematic in several respects. The morbidity burden captured by surveillance data is always an approximation, as individuals may not seek treatment, physicians may not order the required tests for diagnosis, the interpretation of the characteristics of a disease may vary, and a small percentage of all tests result in false positives and false negatives. As well, administrative data sets reflect the policy and procedural contexts of a given jurisdiction, making it difficult to draw comparisons on a national scale. Moreover, morbidity data tend to reflect the most severe illness and that which has already come to the attention of healthcare service providers, though the larger burden of disease may be the cumulative effects of less serious and perhaps remediable conditions in their early stages.
In 1974, a similar observation was made by Marc Lalonde, then Minister of National Health and Welfare. Lalonde’s report, ‘A New Perspective on the Health of Canadians’, challenged the Canadian government to include a fuller spectrum of ill health in population health assessments and to recognize that greater advancements in public health could not be achieved through medical care alone (see text box). Prevention and health promotion efforts advocated by Lalonde would need an evidence base sensitive to a range of health states. Despite the merit of these observations, data on morbidity in Canada are still inadequate to the purposes envisioned by Lalonde in the mid-1970s.
Health-adjusted measures, and the QALY
In the 1960s, consensus grew among many in health and social work that quality of life is a crucial dimension missing in valuations of population health. Added consideration was then given to health gaps—time lived in health states worse than ideal health. Many health gap measures have been proposed (Murray et al., 2000). As Gold et al. explain, Health Adjusted Life Years (HALYs) are a family of measures that combine the impact of death and morbidity, of which Quality Adjusted Life Years (hereafter, QALYs) and Disability Adjusted Life Years (DALYs), which were developed later, are two types (Gold et al., 2002). QALYs take into account both life expectancy and quality of remaining years of life, where health states are the focus. As Murray and colleagues explain, health states measurement requires decisions on which domains of health should be measured (Murray et al., 2000). Within domains, a distinction can be made between performance and capacity, as well as further distinctions between self-reported and observed performance or capacity. The domains commonly measured include each of the senses, pain, mobility, cognition, and more complex domains of social interaction or ability to carry out usual activities (Murray et al., 2000). Health states are weighted according to ratings for health quality between 0 and 1, although negative values can also indicate conditions worse than death. Quality ratings are commonly judged by different groups of experts, including individuals affected, community members, or study investigators. For each domain, the severity-weighted value of time spent in each health state is calculated (Murray et al., 2000). QALYs are used to assess benefits gained from interventions in terms of health-related quality of life and survival. A cost-utility ratio is employed in this assessment; the ratio is defined as the additional cost required to generate one additional year of perfect health (Phillips, 2009). Other descriptive health status measures have been used (Health Utility Index, Health and Activities Limitation Index), and health attributes described differ from one to the next (Gold et al., 2002). Different methods used to elicit ratings of health states (e.g. standard gamble or time trade-offs and rating or visual analogue scales) have raised concerns over inconsistency in values assigned to similar health states or illnesses (Gold et al., 2002).
A New Perspective on the Health of Canadians: The Lalonde Report
The Lalonde report has been credited for a bold observation for its time—that improvements in public health cannot be achieved by biomedical healthcare alone, and population health status cannot be equated with the quality of medical care. Lalonde shifted the vantage point on burden of disease to consider less severe conditions, and by doing so, opened a window discussions of prevention and health promotion.
The 1974 report noted a lack of meaningful assessments of the level of health of Canadians and drew attention to a dependence on indicators of mortality and morbidity. Lalonde noted that major causes of death at this time were due to chronic illnesses and accidents, and that mental illnesses accounted for a large and growing proportion of burden, but that relatively few deaths were due to infectious disease, though influenza and pneumonia were noted as exceptions. With a growing burden of chronic conditions in mind, Lalonde argued the importance of having measures of ill health that would be inclusive of the full range of disability—from mild conditions that were self-managed to more severe conditions requiring medical treatment and hospitalization. He also drew attention to the value of looking at indicators by socio-geographic segments of the Canadian population, noting that greater focus on problems among “high-risk populations” can achieve the most gain. As well, the utility of age-specific and sex-specific analyses were highlighted.
Lalonde advocated for more systematic, comprehensive and sensitive measures of Canadians’ health. Notably, he drew attention to capturing information on burden of disease that is not treated within health care institutions (e.g. outpatient treated burden, self-treated burden, non-severe burden, untreated burden). The author acknowledge that both technical and conceptual problems would need to be resolved in order to measure these burdens, though the conceptual issues were not further detailed (Lalonde, 1974).
Lalonde’s report stands against a traditional backdrop where communicable and non-communicable disease are viewed as dichotomous, mutually exclusive domains. However, as our understanding of complex factors in causation grows (e.g. role of viruses in cancers) and as new challenges arise in the arena of infectious disease control (e.g. antimicrobial resistance and newly emerging infections) the discourse on which elements of disease burden are important to measure may shift yet again.
Health-related quality of life (HRQoL)
HRQoL is a concept that emerged in the 1980s to recognize the effects of disease or disability on health overall and on the quality of life. The Center for Disease Control and Prevention (CDC) has defined HRQoL as “an individual’s or group’s perceived physical and mental health over time”(Centers for Disease Control and Prevention, n.d.), but other authors have adopted a definition that includes three aspects of health: “physical health, e.g. general health, daily functioning, symptoms such as pain, physical disability; mental health, e.g. mood, self-esteem, perception of well-being, perceived stigma; and social health, e.g. social activities and relationships” (see, for example, Lehrner et al., 1999). The intent behind the HRQoL is to develop and use measures that relate to individuals’ perceptions of health as well as their ability to function as they wish in society. This distinguishes HRQoL from measures that strictly assess time lived (mortality or life expectancy) or time lived with or without disability (morbidity), while at the same time, moving away from a utilitarian view of interventions reflected in the QALY.
A number of instruments have been developed to measure HRQoL, (e.g. Medical Outcomes Study Short Forms, the Sickness Impact Profile, and the Quality of Well-Being Scale) and extensively validated in clinical settings or special population studies. Many of the adopted measurement instruments are lengthy, thus incorporating them in regular surveillance has been challenging. However, a CDC initiative sought more compact instruments, which led to the Healthy Days measures, an integrated set of broad questions about perceived health status and activity limitation. The CDC describes other strides made in putting the concept and measures of HRQoL to practical use. For example, the CDC’s Healthy People series of population health progress reports identified improvements to HRQoL associated with chronic disease and risk factors as a public health goal. According to the CDC, a benefit of focussing on HRQoL as a national health standard has been to bridge boundaries between disciplines not traditionally involved in health policy, and between social, mental, and medical service areas (CDC, n.d.). Challenges remain in adequately accounting for diversity in perceptions of health status. Some authors have commented on the need for qualitative research that explores what a good quality of life means to people from different backgrounds, cultures, or other living circumstances (Bounthavong & Law, 2008).
The Global Burden of Disease approach
In the early 1990s, the Global Burden of Disease (GBD) project—initiated by the World Bank in collaboration with the WHO—sought to address several inadequacies seen in health assessment measures. Measures were heavily focused on mortality-based indicators, fragmentary, and inconsistent. Information was not comparable and did not easily support cost-effectiveness analysis (Murray & Lopez, 1999; World Health Organization, n.d.). Finally, many population health assessments did not tie contributing risk factors and exposures to population-level outcomes. The GBD’s objective was to quantify the global burden of premature death, disease, and injury and make recommendations that would improve health, particularly in developing nations (Gold et al., 2002). The first GBD study quantified health effects of over 100 diseases in eight regions of the world, disaggregated by age, sex and region, and introduced a new summary health measure—the DALY (World Health Organization, n.d.).
The GBD is considered a major advance in global health statistics. It allowed comparisons across diseases and world regions and nations, producing internally consistent estimates of disease burden. The inclusion of non-fatal health outcomes was at the forefront of researchers’ concerns and results drew attention toward the importance of chronic, non-communicable diseases in developing nations, as well as the importance of injury and mental illness relative to infectious disease in these regions. As the DALY employed readily available data, the GBD approach offered even developing nations a feasible method of estimating the burden of disease. The WHO officially adopted the burden of disease and DALY approach in the 1990s, supporting nations with lesser capacity for measuring burden. Consensus grew in the 1990s on employing the DALY for the value it held in better supporting comparisons. The study was updated for 2000-02 data to provide more extensive analysis of disease attributable to 26 risk factors, using the Comparative Risk Factor Assessment Framework. In 2010, the Institute for Health Metrics and Evaluation collaborated on a new method for calculating the DALY, which was published in 2012 (World Health Organization, n.d.).
Disability Adjusted Life Years (DALYs)
Like the QALY, the Disability Adjusted Life Year (DALY) is a health adjusted measure of population health status, within the HALY family of summary measures. Gold et al. (Gold et al., 2002) differentiated the two measures in detail, though the most evident difference is that the DALY accounts for quality of life with reference to specific disease categories (applying the International Classification of Impairments, Disabilities and Handicaps). The DALY quantifies morbidity, disabling complications, as well as mortality in a single measure. For a given health condition, the sum of years of life lost to premature death (YLL) in the population are added to the sum of the years lived with disability (YLD) for varying degrees of severity. Severity weights, which are also referred to as disability weights, are used to quantify the reduced quality of life. Panels of experts are responsible for assigning severity weights, which range from 0 for complete health, to 1 for death. The original formulation of the DALY placed different value weights on populations based on their age structure. That is, DALYs in very young and old were discounted relative to other ages (Gold et al., 2002). Calculation of DALYs can incorporate other discounting factors. For example, discount factors are used to value present years of life saved more than future years. Age weighting and discount factors are heavily debated (Knol, et al., 2009). Despite many advantages to the DALY, its proponents concede some particular disadvantages of the measure, including its inherent value judgements on age as well as disability. The DALY also misses downstream effects including, for example, the effects of disease on trade, agriculture and other social, or societal level costs (Navarro, 2009). As well, because the DALY is calculated on a disease-specific basis, it cannot accommodate comorbid conditions, which are important contributors to disease burden. As well, therapies with side-effects cannot be captured within the DALY framework (Gold et al., 2002).
The development of new concepts and approaches to the measurement of burden has provided public health professionals with greater information and insight into population health, although the challenges of applying these ideas in the course of program planning and policy development remain, as will be illustrated in the next section.
Applications of Burden of Disease in Canada
The concept and measures of burden of disease are applied at all levels of public health policy and practice in Canada. NCCID conducted a brief review of Canadian public health documents to determine how burden of disease is currently being applied, framed, and measured in national and sub-national public health program planning and policy initiatives. With a particular focus on infectious diseases, our review revealed a broad scope of meanings, measures, and objectives behind estimating burden of disease in Canada.
At its most basic level, ‘burden of disease’ in public health reports simply characterizes the existence of a disease in a particular population. A number of reports, for example, from the Public Health Agency of Canada (PHAC) describe the burden of various infectious diseases strictly based on estimated incidence and prevalence data (Gallant, et al., 2014). Similarly, the burden of illness estimates for many food-borne illnesses in Canada are based on annual counts of laboratory-confirmed cases from national surveillance systems such as the Canadian Notifiable Diseases Surveillance System (Thomas & Murray, 2014). Case counts and incidence or prevalence rates are very common measures used to represent population disease burden in epidemiologic reports. While these measures are widely used, their accuracy is highly dependent on a variety of factors including choice of denominators, consistency of case definitions, and a jurisdiction’s vigilance for reporting. It is also important to note that the term ‘burden’ is not always referred to in surveillance or epidemiologic reports—even when measures of disease burden are being described (Public Health Agency of Canada, 2009a, 2010, 2014).
Other public health programs and research studies in Canada apply broader meaning and comprehensive approaches to measuring disease burden. They may include measures of disease severity, measures of direct and indirect economic costs, or summary measures of population health (e.g. YLL, HALY, DALY, HALE) (Health Canada, 2014; Kwong, et al., 2010; Betancourt et al., 2014; Public Health Agency of Canada, 2006a). Severity measures such as disease-specific mortality and health care utilization are also widely used measures of disease burden. Common measures of health care utilization in Canada include: disease attributed hospitalization, intensive care unit (ICU) admission, physician visits, and hospital length of stay. Studies estimating the economic burden of particular diseases in Canada use health care utilization measures to determine the direct costs of those diseases to society (Health Canada, 2014; Krueger et al., 2013). The indirect costs of diseases in these studies are determined by estimates of lost production due to premature mortality or morbidity (usually by applying subjective utility or monetary valuations to lost time). Finally, a number of Canadian public health studies utilize summary measures of population health to estimate disease burden more comprehensively (Kwong, et al., 2010; National Advisory Committee on Immunization, 2012). These studies generally use standard methodology and measures to incorporate complex quality of life effects, while allowing for comparisons with national and international populations.
Data availability appears to be a significant factor in defining the scope of measurement for disease burden estimates. The Ontario Burden of Infectious Disease Study report (ONBOIDS, described below) explains that the approach that was taken for burden estimation was driven by the availability of data (Kwong, et al., 2010). However, some initiative is being taken to expand measurements of disease burden. PHAC’s Centre for Food-borne, Environmental and Zoonotic Infectious Diseases plans to increase the scope of their burden measurements beyond simple case counts, to include hospitalizations, deaths and economic costs associated with food-borne illness (Thomas & Murray, 2014). As surveillance systems improve and expand, we might hope to see less reliance on simple measures of incidence and prevalence rates as measures of disease burden in Canada, though purposeful and transparent planning may best assure success.
Numerous public health programs and research projects in Canada have endeavoured to estimate some aspect of disease burden. To exemplify some of the scope and diversity of these projects, and with a focus on infectious disease, NCCID has selected four large Canadian initiatives which have given considered attention to their measures of disease burden for public health decision-making purposes.
HALE /PHAC Strategic Planning
A key population health indicator produced by Statistics Canada to measure health is the health-adjusted life expectancy (HALE). HALE is an indicator of the average number of years that an individual is expected to live in a healthy state given the current morbidity and mortality conditions of the population (Public Health Agency of Canada Steering Committee on Health-Adjusted Life, 2012). It combines both mortality (quantity of life) and morbidity experience (quality of life) into a single summary measure. It can be used to measure not only the burden of disease and injury, but also burden associated with risk factors in the population and the performance of public health efforts. In 2012–13, the Agency published the Health-Adjusted Life Expectancy in Canada: 2012 Report by the Public Health Agency of Canada, which provided HALE estimates for Canadians with and without selected diseases and conditions. Additionally, the report cited studies (McIntosh, et al., 2009) that performed population-level health equity analysis by linking mortality data with income data. These studies indicated that lower levels of neighborhood income and family income were both associated with a significant loss in HALE. Between 2012 and 2014, PHAC adopted the HALE (specifically, HALE at birth and HALE between the top fifth and bottom fifth income groups) as a performance indicator for its primary strategic outcome of “Protecting Canadians and empowering them to improve their health” (Public Health Agency of Canada, 2013). Through these initiatives, the Agency demonstrated their commitment to ensuring that estimates of disease burden used to inform Canadian policy not only incorporate concepts of quality of life, but also accommodate health equity analyses.
EBIC
The Economic Burden of Illness in Canada (EBIC) is a comprehensive cost-of-illness study that began in 1991 to provide objective and comparable information on the magnitude of the economic burden or cost of illness and injury in Canada based on standard reporting units and methods (Health Canada, 2014). The most recent report, EBIC 2005-2008, includes estimates that encompass a number of direct and indirect disease-associated costs, but not measures reflecting intangible costs such as pain and suffering. The three direct cost components estimated were: hospital care expenditures, physician care expenditures, and drug expenditures. Other direct health expenditures (e.g. capital, other professionals, public health, and other health spending) were included in total cost estimates but not attributed to EBIC diagnostic categories. The indirect costs of disease were estimated by the value of lost production due to illness, injury, or premature death associated with time away from labour market activities—they did not include costs associated with presenteeism, non-labour market activities or informal caregiving. The primary objective of EBIC has been to provide robust, economic evidence to support public health policy and program planning in Canada (Health Canada, 2014).
Pandemic Vaccine Prioritization Framework
As an Annex to the Canadian Pandemic Influenza Plan for the Health Sector (CPIP), the Pandemic Vaccine Prioritization Framework has been developed as guidance for vaccine prioritization decisions that must be made if a pandemic were to occur in Canada (e.g. whether or not priority groups are required, and if so, which groups would those be) (Public Health Agency of Canada, 2009b). The Canadian pandemic goals set out in CPIP provide strong direction for decision-making when implementing a pandemic vaccination program. These goals are “First to minimize serious illness and overall deaths, and second to minimize societal disruption among Canadians as a result of an influenza pandemic” (Public Health Agency of Canada, 2006b). The Pandemic Vaccine Prioritization Framework describes a number of considerations that must be made when trying to achieve these goals through vaccine prioritization. The framework emphasizes that understanding the epidemiology of the pandemic and its disease burden is “probably the most important consideration in developing vaccine recommendations, including prioritization of recipients” (Public Health Agency of Canada, 2009b). It highlights several key burden of disease measures for consideration, including pandemic severity, attack rate, and the population groups most affected in terms of mortality and severe morbidity. It also recognizes different ways that mortality or severe morbidity data can be expressed (e.g. number or rate of deaths by age or risk group, years of healthy or working life lost, DALYs). Finally, it notes the ethical dilemma that exists when trying to balance utility (the principle of acting to maximize aggregate welfare) with equity (the fair distribution of benefits and burdens) in vaccine prioritization decisions. The Pandemic Vaccine Prioritization Framework does not try to formalize a weighting system for criteria or measures involved in prioritization decisions. Rather, it uses a formalized structure to characterize the key questions and concerns that should be considered during the decision-making process.
ONBOIDS
The Ontario Burden of Infectious Disease Study (ONBOIDS), completed in 2010, was a large initiative undertaken by the Institute of Clinical and Evaluative Sciences (ICES) and Public Health Ontario (PHO) to describe the relative contributions of certain infectious diseases to the overall burden of infectious diseases in Ontario. It had three other central objectives: to inform priority setting, planning, and decision-making; to establish a baseline for future evaluations of public health interventions; and to identify the strengths and weaknesses of existing data on infectious diseases (Kwong, et al., 2010). The investigators used a HALY as their primary measure of disease burden, though they tailored their approach and declared certain value-based decisions made in calculating their HALY (such as using a local life expectancy value instead of a global one, and not including age weighting in their analysis) which may have made their results less comparable to other burden of disease studies worldwide. The authors highlight two other important limitations in their report. First, they explain that their methods did not allow for an evaluation of the full impact of infectious disease outbreaks on the broader economy or society. They express a need for novel methods to comprehensively assess the health and economic burden of infectious disease outbreaks in Canada. Second, they underscore the fact that their study did not include an assessment of the burden of disease prevented by successful interventions such as vaccination programs, early antibiotic treatment, or hospital infection control. They note that the low burden of disease associated with vaccine-preventable diseases in Canada could mostly be attributed to the success of vaccination programs and should not diminish the attention that they receive from public health. ONBOIDS is one of the most thorough examinations of the burden of infectious diseases in Canada to date. However, as the authors note, data quality and availability had a significant effect on the extent of analyses they could perform. Any efforts to broaden the scope of disease burden estimation in Canada may, therefore, need to proceed in step with commitments to improve data and surveillance infrastructure.
This review demonstrates an incredibly wide scope of purposes, measurements, and meanings behind burden of disease estimations in Canadian public health program planning and policy initiatives. However, many of these initiatives appear to espouse a similar purpose, that is, to support public health policy and planning through disease monitoring and prioritization. Whether it’s to develop strategic outcomes and performance indicators for PHAC, create a national pandemic vaccine prioritization framework, describe the economic cost of illness and injury in Canada, or determine the relative contribution of select infectious (or non-infectious) diseases to overall disease burden, accurate and comprehensive burden of disease estimates are essential for progress and positive outcomes.
Gaps, Questions and Considerations toward a Framework
This concept paper has brought to mind a range of burden-related terms in common usage, some important milestones in conceptualizing and measuring burden, and examples of current applications of burden measures in Canadian public health program planning and policy initiatives. By now, some omissions and shortcomings may be apparent. In this section, the aim is to begin to identify gaps and raise critical questions concerning burden and its measurement to consider as we set goals for developing clearer concepts and a broader framework by which we can understand important dimensions of disease burden, particularly infectious disease burden. Such a framework could help public health decision-makers to be aware of the potential scope of burden and to be conscious of their own usage. It can be employed to assess the relevance of additional elements of burden for the purposes of public health programmers and policy makers, and to consider the feasibility of measuring or estimating them. At this preliminary, conceptual stage, the objective is to question, provoke ideas, and invite input from diverse public health stakeholders, whereas the longer-range objective is to develop a practical framework that serves the needs of decision-makers and the populations they serve.
Go wide, go deep? Is there room for different approaches?
Ian McDowell, author of Measuring Health, observed a trend in health measurement toward the use of a narrower range of instruments and consolidated methods for measuring burden (Mcdowell, 2006). Certainly, the Global Burden of Disease project has stimulated a great deal of research centred on the use of the DALY as a summary measure, which was regarded as a significant advancement for the measurement of burden for reasons already described. Yet, as mentioned, the limited attention given to definitions of burden of disease compared to definitions of DALYs and accounts of the GBD project may suggest that the concept of burden is being eclipsed by this specific approach. It is unlikely that any one summary measure of disease burden can meet all objectives of health assessment, nor account for the effects of different diseases or injuries, and at the levels of analysis that may be of interest. As noted by Thacker et al. (Thacker, 2006), burdens by different measures and data sources give a very disparate picture of population health priorities. For example, a top ten ranking of health conditions by different measures of burden based on US national statistics showed influenza and pneumonia ranked 7th among causes of death, but these conditions do not appear among the most costly conditions, or among those that contribute most to DALYs, or YPLL. The differences are understood to reflect a combination of disease characteristics and the focus of various measures. Although it is clear that rankings are over-simplifications for most purposes, the larger point made by Thacker et al. concerns the limitation of single measures of burden and that consensus is not likely achievable (Thacker, 2006). According to a US Institute of Medicine panel convened to review summary measures, all measures of population health involve choices and value judgements in both their construction and their application. The panel recommended open discussion of assumptions associated with the approaches taken, as well as establishment of standards and investment in training (Murray et al., 2000).
How are assumptions and uncertainties addressed in measuring disease burden?
According to Knol et al., uncertainties and assumptions behind estimates of environmental burden of disease are not often made clear, yet policy makers need to understand the sources and implications of uncertainty to make better decisions. The authors suggest the use of a typology to help identify uncertainties, communicate across the multiple disciplines involved in environmental public health, and interpret uncertainties for how they affect the utility of health assessment results in supporting decision-making. Though they concede the merits of summary measures, like the DALY, the authors caution that policy makers must take responsibility for understanding assumption and uncertainties inherent to measures. Knol et al. cite examples of four-fold variation in DALYs that resulted from different assumptions and illness severity weights being applied, confirming a need for caution in the use of such measures for policy purposes (Knol et al., 2009). Uncertainties and assumptions particular to infectious disease health assessments might also be explored.
What place do qualitative research methods and subjective assessments of burden have?
Because improvements in biological outcomes for a health condition are not necessarily accompanied by similar improvements in well-being perceived by those affected, some assessments of disease burden have considered subjective measures of health-related quality of life. However, the ‘quality’ construct is complex and challenging to measure and analyse. As already noted, many quality of life scales have been developed and validated. These quantitative measures provide a convenient means of simplifying complex information on subjective experiences, summarizing and analysing information drawn from large population samples using statistical methods. However, it may be difficult to capture factors felt to be important by subjects with closed-ended questions and a necessarily limited, ordered set of answers. The potential for error may be great. Qualitative methods (e.g. interviews and case studies) and data add rich contextual information about patient-defined disease outcomes. It may be important to understand these experiences and perceptions in depth, not only in terms of the consequences of disease for individuals, families, and communities, but also for the information that may be gleaned about relevant and acceptable strategies to reduce burden.
What levels of infectious disease burden are considered, and what etiological framework is applied?
It is important to consider whether burden of disease has been adequately accounted for at all levels at which health conditions have significant costs and consequences, that is, not only for the individual, but within families and other key social relationships, the community, at the health and public health systems level, and at a societal level. More importantly, public health professionals would do well to consider the etiological framework in which they view these burdens. Is the burden of disease attributed to decontextualized individual behaviours or is a structural model of causation adopted, with a view to the contexts and processes in which disease originates? Pearce (Pearce, 1996) raised this as a concern for trends in epidemiology in a 1996 issue of the American Journal of Public Health, and it remains a salient point. The author described a shift in epidemiology from a population perspective to a reductionist approach focused on an individual level of analysis. According to the author, “epidemiology has largely ceased to function as part of a multidisciplinary approach to understanding the causation of disease in populations and has become a set of generic methods for measuring associations of exposure and disease in individuals”. Pearce went on to level criticism at the field for neglecting social, economic, cultural, historical, political, and other population level contexts.
How relevant are measures of burden to a particular level of public policy development?
Authors of an Estonian study considered trade-offs of internationally comparable results from a GBD approach to burden versus local relevance, and opted to tailor their strategy to serve their national and local policy needs. The authors found that by contextualizing the measure they achieved added value and favourable uptake of results by policy makers. To contextualize the measure, they used disease classification corresponding to an Estonian disease profile, made use of routinely collected data in Estonia, and used national disease severity assessments in disability weights. The authors also adjusted goals to not only reduce overall burden of disease but also decrease inequities and improve health-related quality of life (Lai et al., 2009). As mentioned earlier, ONBOIDS researchers took a very similar ‘localized’ approach to measuring disease burden in Ontario. These examples may be illustrative for those adopting the GBD approach and measures, although critical consideration of the applicability of any health assessment method and measure to a specific policy context may be important.
How can the concept and measures of burden be expanded beyond medically defined deficits?
Researchers at the US CDC have described the common approach to burden as being narrow and overly focused on mortality and morbidity (Centres for Disease Control and Prevention, n.d.). A biomedical paradigm is seen to be dominating much of the discourse on burden. Many assessments of population health still define health simply on the basis of clinical features and only adverse health effects that are diagnosed by medical doctors. According to Thacker et al., the WHO definition of health, “health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity”, has long been in use, and yet the positive and social dimensions it refers to are not accounted for by burden measures (Thacker, 2006). Only negative aspects are quantified. Thacker et al. suggest there is a need to put attention to aspects of health that reflect the quality of life to which people aspire, the activities that protect public health, and social capital or health-supporting conditions and relationships. Writing in 2006, the authors note the CDC’s intentions at that time to further explore these non-traditional measures of burden.
Is the importance of reducing inequitable burden being addressed?
In a commentary piece, David Mowat stated that the measurement of the overall health status of the Canadian population hides serious disparities, such as an eight-fold higher prevalence of tuberculosis in Aboriginal Canadians. Mowat went on to emphasize the important role public health has in reducing health disparities (Mowat & Butler-Jones, 2007). The view echoes a WHO discussion paper on approaches to social inequities in health, which suggested that population health policies should have the dual purpose of promoting health gains in the population as a whole and reducing health inequities (Whitehead, 1992). Gold and colleagues have drawn attention to an unresolved ethical dilemma that some summary measurers of health (e.g. QALYs), having been shaped by utilitarian social theory, work on the assumption that policies should do the greatest good for the largest number of people (Gold et al., 2002). However, greater equity may require doing the most good for only some who carry the heaviest burden of disease. An added challenge for reducing infectious disease inequities may be that studies in this area have not received the same prominence as studies on some chronic diseases (e.g., Marmot, 2004).
How can structural / upstream factors to which disease burden is attributable be better accounted for?
Upstream, structural and social determinants of health may be further explored for infectious diseases. For example, the underlying determinants of influenza burden in remote and isolated First Nations communities could be more fully understood as consequences of low-quality housing, crowded living conditions, high exposure to indoor air pollutants, lack of access to critical infrastructure, higher prevalence of predisposing health conditions and co-morbidities (Moghadas, 2014). The influence of food insecurity might also be considered. As well, there is a need to better understand determinants that influence susceptibility to severe illness, an area which calls for greater attention from public health decision-makers.
Nearly 15 years ago, CDC invested in research on non-traditional measures of burden, moving upstream to the social and environmental conditions which shape risks for disease or injury. According to Thacker and colleagues, some measures exist in this area, but they are still limited. Writing in 2006, the authors note that the factors being explored include social capital, social relationships, social cohesion, healthy peer relationships, and school or work environments supportive of good health. Further work remains to assess the feasibility of developing appropriate metrics of burden in these areas (Thacker et al., 2006).
Looking downstream of common burden measures, what more can be done to include information on co-morbid conditions?
Accounting for co-morbid conditions in measures of disease burden presents challenges. Taking the example of the EBIC, the report’s authors point out that hospital care expenditures were not attributed to comorbid conditions, leading to under-estimates for certain conditions. The authors concede that discharge abstracts for the majority of hospital databases do contain information on comorbid conditions, and suggest that future editions could weight expenditures according to primary and comorbid conditions.
How might the severity of infectious disease burden be better accounted for?
Certain summary measures of health (e.g. HALYs) have incorporated information on severity by applying a severity weighting factor to the time lived with particular health conditions. However, awareness of the subjective nature of judgements of severity has raised questions about who is the most appropriate judge of disease severity. Are clinicians or other experts the best judges of severity, or are those who are affected by the disease or injury the rightful judge of the experience? Some have suggested that representative sampling could provide a more fair assessment of severity.
Overall, many assessments of disease burden scarcely address severity measures, which at least in part reflects shortcomings in surveillance systems. For influenza, incidence rates or attack rates are often still the major focus of health assessment rather than indicators of severe illness such as hospital separation, hospital days, ICU admission, or death.
Do we have sufficient information about the burden of infectious disease in the aging and elderly?
Some approaches to the measurement of disease burden have given less importance to consequences for older and elderly people. Yet research from the fields of geriatric medicine and gerontology draw attention to considerable variability in the aging process and preventability of disease and disability in older age. Given concerns that Canada’s population is aging and that costs of care will escalate, it may be increasingly important to make health assessment more sensitive to burden in older age groups in order to better understand how public health can intervene for improved quality of health. For infectious diseases, aging with HIV has become one priority area for understanding the potential for greater gains in quality of life. Influenza is associated with serious debilitating complications in elderly individuals and greater focus on issues like vaccine effectiveness for those aged 65 and older, and attaining longer term immune memory for vaccine in this group requires added attention to burden of influenza in this population.
How can gender bias in assessment of infectious disease burden be addressed?
As the EBIC report details, indirect costs of illness to men tend to be much larger than for women, although as the authors suggest, this is likely to reflect omissions in the measure and data sources. Neither lost production from unpaid work nor for the care of others who are sick or injured are counted, which leads to a systematic bias in the measurement of burden for women who contribute more time to these activities. As well, though women actually miss more days of work (i.e. paid labour) due to illness than men, women’s lower earnings outweigh the effects of greater amounts of sick time on measures of lost production (Health Canada, 2014). A dual burden is also implied, as women disproportionately experience income inadequacy and their lesser earnings make them more vulnerable to lost income in circumstances where they care for others who are ill.
Has the burden of infectious disease for caregivers been adequately addressed?
Unpaid, informal caregiving represents a significant though commonly unacknowledged contribution to the Canadian economy and to public health, yet the burden of caring for those who are ill and injured, as well as the burden for recipients of care consequent to caregivers’ illness, are not commonly accounted for. Physical, emotional, social, and financial burdens accrue to those who provide unpaid care, though several factors are recognized as having a mediating influence on caregiving burden, and rewards of caregiving have also been acknowledged (Amstrong & Kit, 2004). The relationship between caregiving and risk for infectious disease has been explored in research, including a study by Kiekolt-Glaser et al., who found that older caregivers had significantly poorer immune responses to influenza virus vaccine than controls (similar for vaccine history, age, income, and chronic disease), which is consistent with experimental studies on stress-related modulation of immunity (Kiecolt-Glaser et al., 1991). Bringing a gender-based analysis to bear, some researchers have also raised critical questions about the effects that downsizing and privatizing healthcare has had on predominantly female caregivers, including greater demands and deterioration of conditions in which unpaid (and paid) care is provided (Armstrong et al., 2001). Thus, tracking caregiving burden requires information on both population health status (e.g. how many people require informal care in recuperating from influenza and its complications or how many lack care owing to a caregiver’s illness) and a health resource burden that may risk care being offloaded to informal providers (e.g. emergency services overload during peak attack rates of pandemic influenza).
Has the burden of public health interventions been accounted for?
Public health interventions and strategies have their own costs, which may not adequately be considered. For example, school closures have detrimental social and economic consequences. Some factors include: missed school work, rearranging household schedules, difficulty making alternate childcare arrangements, decreased productivity in the workforce, decreased capacity in the health care system if professionals stay home to care for their children, lost income, fear of infection, and the cost of childcare (Isfeld-Kiely & Moghadas, 2014). Moreover, the appropriateness and effectiveness of medical treatment or interventions cannot be assumed to be uniform for the recipients of services, and may differ on the basis of biological characteristic like sex of the individual, but particularly by social factors, including gender, ethnicity/cultural group, rural or remote residence, or income level.
How can we account for ‘prevented burden’ and public health successes?
According to Thacker et al., the prevention and health protection roles of public health (e.g. immunization) are important, and yet burden, as a deficit measure cannot estimate their effects (Thacker et al., 2006). Curry et al. provide as an example that where burden of disease is low, but has been achieved through effective public health measures, sustained or increased funding may be needed to maintain capacity and prevent a resurgence of disease. Successful programs are important dimensions to consider in prioritizing public health intervention and they need place markers in the data and policy conversations. The authors call for an expanded repertoire of measures to reflect public health roles in prevention and health protection (Curry et al., 2006). The authors of the ONBOIDS report raise the same point and suggest that an important complementary study to theirs would involve an investigation into the number of lives saved and morbidity prevented through various intervention strategies such as vaccination (Kwong, et al., 2010).
Conclusion & Next Steps
In this discussion paper, NCCID has begun to plumb and map the conceptual construct of burden of disease, with aims to build understanding of the depth and breadth of meaning that has accrued to the term, and to explore some current applications in research and in program and policy development. Burden of disease measurement holds much promise of practical benefits for prioritizing public health action on burden-producing problems, guiding the use of scarce resources to the best effect, and furthering goals for greater equity in health outcomes. Yet its potential is unlikely to be realized without greater collaboration among disciplines and a more integrated, systematic, and transparent approach.
There has been a proliferation of burden-related terms in the academic literature. These terms allude to wide ranging dimensions of burden, though definition and validation of measures may be lacking. Social dimensions of burden do not receive the same attention as biomedical outcomes, and appear more often in discussions of particular disease categories or risk factors. They do not appear to be integrated within larger-scale, multi-disease initiatives. Structural determinants of health and equity considerations are not prominent in the discourse on burden of disease, whereas consolidation of methods and metrics appear to be the greater focus.
Over time, key concepts and methods for measurement have developed, which have helped apply disease burden information toward practical ends. Though each approach has limitations and omissions, their critical appraisal has propelled improvements in data collection and analysis. Refinements in this work has led to important revelations about disease trends and overlooked costs of chronic, non-communicable diseases.
Although this review was admittedly limited in coverage, it suggests that a wide range of methods are currently applied in estimating disease burden in program and policy initiatives. Many projects are limited to the use of simple case counts. The focus is often on individual diseases or risk factors, though the scope of measurement may vary according to the policy level to which the work relates. Mortality-based measures still predominate, and a biomedical perspective persists. The GBD approach adds an adjustment for time lived with disability to the equation, allows for comparison across diseases and regions, and supports analysis by contributions of major risk factors. Yet its orientation to factors important to health in developing nations raises questions for its utility for prioritizing Canadian health issues. Overall, the measurement of disease burden remains siloed and piecemeal.
From this preliminary review, it appears that the objectives of burden of disease assessment would be better served by explicit definition of the overarching concept and specificity of the purpose to which burden assessment is being applied. There is often little sense of whether the purpose of a burden of disease initiative has been served, whether good uptake of the information has been achieved, and how that information is subsequently acted upon. As well, there is a need for expansion of the burden concept beyond the purview of biomedicine. This may begin with greater awareness of various dimensions of burden that have been explored by allied population health disciplines, and of other levels at which consequences of an individual’s disease occur (i.e. family, social, community, or societal levels). While there has been some innovation in non-traditional measurement of burden (e.g. social capital), efforts to track progress and publicize the work may have lapsed.
As a long-range goal, the aim of this foundational work is to develop a fully articulated framework to guide burden of disease measurement and prioritization of public health decision-making, particularly in the area of infectious disease public health. To this end, NCCID invites population and public health stakeholders to consider the ideas and questions raised within this discussion paper and to contact us with input. Specific mechanisms have also been planned to solicit broad input, including the development of an advisory group and a workshop event. Consultation with population public health professionals assures a more practical and relevant approach.
Production of this document has been made possible through a financial contribution from the Public Health Agency of Canada through funding for the National Collaborating Centres for Public Health (NCCPH).
The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada. Information contained in the document may be cited provided that the source is mentioned.
References
Adhikari, S. R., Maskay, N. M., and Sharma, B. P. (2009). Paying for hospital-based care of Kala-azar in Nepal: Assessing catastrophic, impoverishment and economic consequences. Health Policy and Planning, 24(2), 129–139. http://doi.org/10.1093/heapol/czn052
Ahrens, W., and Pigeot, I. (2007). Handbook of Epidemiology. Springer Science and Business Media. Retrieved from https://books.google.com/books?id=Szmnh7R1RQQC&pgis=1
American Diabetes Association. (2013). Economic costs of diabetes in the U.S. in 2012. Diabetes Care, 36(4), 1033–46. http://doi.org/10.2337/dc12-2625
Amstrong, P. and Kit, O. (2004). One hundred years of caregiving. In K. Grant, Karen R., Amaratunga, C., Armstrong, P., Boscoe, M., Pederson, A., Willson, K. (Ed.), Caring for / Caring about: Women, home care, and unpaid caregiving. (pp. 45–73). Aurora, Ontario: Garamond Press.
Ariana, P. (2012). Challenging our understanding of health: Indigenous perspectives from the highlands of Chiapas, Mexico. Oxford Developmental Studies, 40(3), 405–421. Retrieved from http://search.proquest.com.libproxy.uwinnipeg.ca/socabs/docview/1322723222/888938C284CD47C4PQ/20?accountid=15067
Armstrong, P., Amaratunga, C., Bernier, J., Grant, K., Pederson, A., Willson, K. (2001). Exposing Privatization: Women and Health Care Reform in Canada. (Armstrong, P, Amaratunga, C., Bernier, J., Grant, K., Pederson, A., Willson, K. Ed.). Aurora, Ontario: Garamond Press. Retrieved from https://books.google.com/books?hl=en&lr=&id=royYtCZGNegC&pgis=1
Aubert, L., Pichierri, S., Hommet, C., Camus, V., Berrut, G., and de Decker, L. (2015). Association between comorbidity burden and rapid cognitive decline in individuals with mild to moderate Alzheimer’s disease. Journal of the American Geriatrics Society, 63(3), 543–547. http://doi.org/10.1111/jgs.13314
Barry, J. A. (2014). Caregiving men of Alzheimer’s disease sufferers in Nuevo Leo’n (Mexico) experiences and meanings, Vulnerable Groups and Inclusion (5), 1–23. Retrieved from: http://www.vulnerablegroupsandinclusion.net/index.php/vgi/article/view/24166
Betancourt, M. T., Roberts, K. C., Bennett, T-L., Driscoll, E. R., Jayaraman, G., and Pelletier, L. (Spring 2014). Monitoring chronic diseases in Canada: the Chronic Disease Indicator Framework. Chronic Diseases and Injuries in Canada, 34(Suppl. 1)1-30. Retrieved from: http://www.phac-aspc.gc.ca/publicat/hpcdp-pspmc/34-1-supp/assets/pdf/34-S1_E_v6.pdf
Bolton, S. and Talman, A. (2010). Interactions between HIV/AIDS and the Environment: A Review of the Evidence and Recommendations for Next Steps. Nairobi, Kenya: IUCN ESARO Office. viii + 62pp.
Bounthavong, M., and Law, A. V. (2008). Identifying health-related quality of life (HRQL) domains for multiple chronic conditions (diabetes, hypertension and dyslipidemia): patient and provider perspectives. Journal of Evaluation in Clinical Practice, 14(6), 1002–11. http://doi.org/10.1111/j.1365-2753.2007.00933.x
Boutayeb, A. (2006). The double burden of communicable and non-communicable diseases in developing countries. Transactions of the Royal Society of Tropical Medicine and Hygiene, 100(3), 191–199. http://doi.org/10.1016/j.trstmh.2005.07.021
Centers for Disease Control and Prevention. (1986). Premature mortality in the United States: Public health issues in the use of years of potential life lost. MMWR. Morbidity and Mortality Weekly Report, 35(2 Suppl), 1S–11S. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3097485
Centers for Disease Control and Prevention. (n.d.). CDC – Concept – HRQOL. Retrieved April 30, 2015, from http://www.cdc.gov/hrqol/concept.htm
Cuijpers, P., Smit, F., Unger, F., Stikkelbroek, Y., Ten Have, M., and de Graaf, R. (2011). The disease burden of childhood adversities in adults: a population-based study. Child Abuse & Neglect, 35(11), 937–45. http://doi.org/10.1016/j.chiabu.2011.06.005
Curry, C. W., De, A. K., Ikeda, R. M., and Thacker, S. B. (2006). Health burden and funding at the Centers for Disease Control and Prevention. American Journal of Preventive Medicine, 30(3), 269–276. http://doi.org/10.1016/j.amepre.2005.10.028
Dempsey, M. (1947). Decline in tuberculosis: death rate fails to tell entire story. Am Rev Tuberculosis, 56, 157–64.
Donovan, D., McDowell, I., Hunter, D. (n.d.). Glossary. Retrieved April 30, 2015, from http://www.afmc-phprimer.ca
Englisch, S., Esser, A., Enning, F., Hohmann, S., Schanz, H., Zink, M. (2010). Augmentation with pregabalin in schizophrenia. Journal of Clinical Psychopharmacology, 30(4), 437.
Entsua-Mensah, K., Doku, A., and Adzamli, I. (2012). The National Cardiothoracic Centre, Accra Ghana: proceedings of the second International Update Course in Cardiology – improving the coverage of cardiology services. The Pan African Medical Journal, 11, 8
Fogarty, E., Walsh, C., McGuigan, C., Tubridy, N., Barry, M. (2014). Direct and indirect economic consequences of multiple sclerosis in Ireland. Applied Health Economics and Health Policy, 12(6), 635.
Gallant, V., McGuire, M., Ogunnaike-Cooke, S. (2015). A summary of tuberculosis in Canada, 2013. CCDR, 41 S(2), 8–15. Retrieved from http://phac-aspc.gc.ca/publicat/ccdr-rmtc/15vol41/dr-rm41s-2/editorial-eng.php
Gold, M. R., Stevenson, D., and Fryback, D. G. (2002). HALYS and QALYS and DALYS, Oh My: Similarities and differences in summary measures of population Health. Annual Review of Public Health, 23, 115–134. http://doi.org/10.1146/annurev.publhealth.23.100901.140513
Health Canada. (2014). Economic burden of illness in Canada. Government of Canada, Ottawa, Canada. Retrieved from http://publications.gc.ca/collections/Collection/H21-136-1998E.pdf
Isfeld-Kiely, H. and Moghadas, S. (2014). Effectiveness of school closure for the control of influenza; a review of recent evidence. Winnipeg, Manitoba: National Collaborating Centre for Infectious Diseases.
Jones, C. O. H., and Williams, H. A. (2004). The social burden of malaria: What are we measuring? Am J Trop Med Hyg, 71(2_suppl), 156–161. Retrieved from http://www.ajtmh.org/content/71/2_suppl/156.short
Kiecolt-Glaser, J. K., Dura, J. R., Speicher, C. E., Trask, O. J., and Glaser, R. (1991). Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosomatic Medicine, 53(4), 345–62. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1656478
Knol, A. B., Petersen, A. C., van der Sluijs, J. P., and Lebret, E. (2009). Dealing with uncertainties in environmental burden of disease assessment. Environmental Health : A Global Access Science Source, 8, 21. http://doi.org/10.1186/1476-069X-8-21
Kobeissi, L., Araya, R., Kak, F., Ghantous, Z., Khawaja, M., Khoury, B., … Zurayk, H. (2011). The relaxation exercise and social support trial-rest: Study protocol for a randomized community based trial. BMC Psychiatry, 11(1), 142. http://doi.org/10.1186/1471-244X-11-142
Krueger, H., Noonan, V. K., Trenaman, L. M., Joshi, P., and Rivers, C. S. (2013). The economic burden of traumatic spinal cord injury in Canada. Chronic Diseases and Injuries in Canada, 33(3), 113–122.
Kwong, J.C., Crowcroft, N. S., Campitelli, M. A., Ratnasingham, S., Daneman, N., Deeks, S. L., Manuel, D. G., O. B. of I. D. S. A. G. (2010). Ontario Burden of Infectious Disease Study. Toronto, Ontario: Ontario Agency for Health Protection and Promotion; Institute for Clinical Evaluative Sciences. https://www.publichealthontario.ca/en/eRepository/ONBoID_ICES_Report_ma18.pdf
Lai, T., Habicht, J., and Kiivet, R. a. (2009). Measuring burden of disease in Estonia to support public health policy. European Journal of Public Health, 19(5), 541–547. http://doi.org/10.1093/eurpub/ckp038
Lajoie, J. (2015). Understanding the Measurement of Global Burden of Disease. Winnipeg: National Collaborating Centre for Infectious Diseases.
Lalonde, M. (1974). A new perspective on the health of Canadians. Vasa, 76. http://doi.org/H31-1374
Lehrner, J., Kalchmayr, R., Serles, W., Olbrich, a, Pataraia, E., Aull, S., … Baumgartner, C. (1999). Health-related quality of life (HRQOL), activity of daily living (ADL) and depressive mood disorder in temporal lobe epilepsy patients. Seizure : The Journal of the British Epilepsy Association, 8(2), 88–92. http://doi.org/10.1053/seiz.1999.0272
Lennon, D., Reid, S., Stewart, J., Jackson, C., Crengle, S., and Percival, T. (2012). Reducing inequalities with vaccines: New Zealand’s MeNZB vaccine initiative to control an epidemic. Journal of Paediatrics and Child Health, 48(3), 193–201. http://doi.org/10.1111/j.1440-1754.2010.01969.x
Lim, S. S., Vos, T., Flaxman, A. D., Danaei, G., Shibuya, K., Adair-Rohani, H., … Memish, Z. A. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380(9859), 2224–60. http://doi.org/10.1016/S0140-6736(12)61766-8
Link, B. G., and Phelan, J. (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, Spec No, 80–94. http://doi.org/10.2307/2626958
Maji, KR, Sood, M., Sagar, R., Khandelwal, S. K. (2012). A follow-up study of family burden in patients with bipolar affective disorder. The International Journal of Social Psychiatry, 58(2), 217. http://doi.org/10.1177/0020764010390442
Malinowsk, K.P., Kawalec, P. (2015). The indirect costs of ankylosing spondylitis: A systematic review and meta-analysis. Expert Review of Pharmacoeconomics & Outcomes Research, 15(2), 285–300.
Marmot, M. (2004). Status syndrome. Significance, 1(4), 150–154. http://doi.org/10.1111/j.1740-9713.2004.00058.x
May, C. R., Eton, D. T., Boehmer, K., Gallacher, K., Hunt, K., MacDonald, S., … Shippee, N. (2014). Rethinking the patient: Using Burden of Treatment Theory to understand the changing dynamics of illness. BMC Health Services Research, 14(1), 281. http://doi.org/10.1186/1472-6963-14-281
McDowell, I. (2006). Measuring Health : A Guide to Rating Scales and Questionnaires. Oxford University Press.
McIntosh, C.N., Fines, P.,Wilkins, R., Wolfson, M. C. (2009, December). Income disparities in health-adjusted life expectancy for Canadian adults, 1991-2001. Health Reports, 20(4), 55–64.
Moghadas, S. (2014). Optimal treatment strategies for remote and isolated communities. Purple Paper, (41), 1–3. National Collaborating Centre for Infectious Diseases.
Mowat, D. L., and Butler-Jones, D. (2007). Public health in Canada: A difficult history. Healthcare Papers, 7(3), 31–36.
Muennig, P., Franks, P., Jia, H., Lubetkin, E., and Gold, M. R. (2005). The income-associated burden of disease in the United States. Social Science & Medicine (1982), 61(9), 2018–26. http://doi.org/10.1016/j.socscimed.2005.04.005
Murray, C. J. L., Salomon, J., and Mathers, C. (2000). A critical examination of summery measures of population health. Bulletin of the World Health Organisation, 78(8), 981–994. http://doi.org/ok não usei
Murray, C. J., and Lopez, A. (1999). On the comparable quantification of health risks: Lessons from the Global Burden of Disease Study. Epidemiology (Cambridge, Mass.), 10(5), 594–605.
National Advisory Committee on Immunization. (2012). Update on Human Papillomavirus (HPV) vaccines. Canada Communicable Disease Report, 38(January), 1–62.
Navarro, C. (2009). Global burden of food-borne disease. CMAJ : Canadian Medical Association Journal, 180(8), 808.
Oxford English Dictionary (1995). The Concise Oxford Dictionary: Ninth Edition. Clarendon Press.
Pearce, N. (1996). Traditional epidemiology, modern epidemiology, and public health. American Journal of Public Health, 86(5), 678–683. http://doi.org/10.2105/AJPH.86.5.678
Phillips, C. (2009). What is a QALY? What Is…? Series. 2nd Edition. Hayward Group plc. Retrieved from http://www.medicine.ox.ac.uk/bandolier/painres/download/whatis/qaly.pdf
Public Health Agency of Canada. (2006a). Calgary based study of influenza vaccination for young children: parental beliefs and behaviours – Canada Communicable Disease Report – Vol. 32-13 – Public Health Agency of Canada. Retrieved from http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/06vol32/dr3213a-eng.php
Public Health Agency of Canada. (2006b). The Canadian Pandemic Influenza Plan for the Health Sector – Influenza – Public Health Agency of Canada. Retrieved from http://www.phac-aspc.gc.ca/cpip-pclcpi/index-eng.php
Public Health Agency of Canada. (2009a). Epidemiology of Mumps in Canada, Volume 35-. Retrieved from http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/36s1/emc-eoc-eng.php
Public Health Agency of Canada. (2009b). Pandemic Vaccine Prioritization Framework – Public Health Agency of Canada. Retrieved from http://www.phac-aspc.gc.ca/cpip-pclcpi/vf/index-eng.php
Public Health Agency of Canada. (2010). FluWatch – Public Health Agency of Canada. Retrieved from http://www.phac-aspc.gc.ca/fluwatch/index-eng.php
Public Health Agency of Canada. (2013). Report on plans and priorities 2013-2014. Ottawa, Ontario: Public Health Agency of Canada.
Public Health Agency of Canada. (2014). HIV and AIDS in Canada: Surveillance Report to December 31st, 2013 – Public Health Agency of Canada. Retrieved from http://www.phac-aspc.gc.ca/aids-sida/publication/survreport/2013/dec/index-eng.php
Public Health Agency of Canada. (2015). Hepatitis B in Canada: 2005-2011 Surveillance Report – Public Health Agency of Canada. Retrieved from http://phac-aspc.gc.ca/sti-its-surv-epi/hepb/surv-eng.php
Public Health Agency of Canada Steering Committee on Health-Adjusted Life. (2012). Health Adjusted Life Expectancy in Canada. Ottawa, Ontario: Public Health Agency of Canada. Retrieved from http://www.phac-aspc.gc.ca/rpp/2013-2014/index-eng.php
Rivera-Navarro, J., Morales-González, J. M., and Benito-León, J. (2003). Informal caregiving in multiple sclerosis patients: data from the Madrid Demyelinating Disease Group study. Disability and Rehabilitation, 25(18), 1057–1064. http://doi.org/10.1080/0963828031000137766
Sav, A., McMillan, S. S., Kelly, F., Kendall, E., Whitty, J. A., King, M. A., and Wheeler, A. J. (2012). Treatment burden among people with chronic illness: What are consumer health organizations saying? Chronic Illness, 9(3), 220–232.
Sav, A., Kendall, E., McMillan, S. S., Kelly, F., Whitty, J. A., King, M. A., and Wheeler, A. J. (2013). “You say treatment, I say hard work”: Treatment burden among people with chronic illness and their carers in Australia. Health and Social Care in the Community, 21(6), 665–674. http://doi.org/10.1111/hsc.12052
Shane, A, Hiebert, J, Sherrard, L, Deehan, H. (2014, June 12). Measles surveillance in Canada: Trends for 2013. CCDR. Public Health Agency of Canada. Retrieved from http://phac-aspc.gc.ca/publicat/ccdr-rmtc/14vol40/dr-rm40-12/dr-rm40-12-surv-1-eng.php
Sills, M. R., Hall, M., Simon, H. K., Fieldston, E. S., Walter, N., Levin, J. E., … Shah, S. S. (2011). Resource burden at children’s hospitals experiencing surge volumes during the spring 2009 H1N1 influenza pandemic. Academic Emergency Medicine : Official Journal of the Society for Academic Emergency Medicine, 18(2), 158–166. http://doi.org/10.1111/j.1553-2712.2010.00992.x
Starfield, B. (2001). Glossary, Basic concepts in population health and health care. J. Epidemiol Community Health, 55, 452–454.
Szucs, T. (1999). The socio-economic burden of influenza. The Journal of Antimicrobial Chemotherapy, 44 Suppl B, 11–15.
Taylor, G., Mitchell, R., McGeer, A., Frenette, C., Suh, K. N., Wong, A., … Gravel, D. (2014). Healthcare-associated influenza in Canadian hospitals from 2006 to 2012. Infection Control and Hospital Epidemiology, 35(2), 169–75. http://doi.org/10.1086/674858
Thacker, S.B., Stroup, D, F., Carande-Kulis, V., Marks, J.S., Roy, K., and Gerbeding, J.L. (2006). Measuring the public’s health, Public Health Reports,12(1), 14–22.
Thomas, M.K., Murray, R. (2014, August 14). Estimating the burden of food-borne illness in Canada. CCDR. Public Health Agency of Canada. Retrieved from http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/14vol40/dr-rm40-14/dr-rm40-14-comm-eng.php
Vella, S.-L., and Pai, N. (2013). The measurement of burden of care in serious mental illness: A qualitative review. The Australian and New Zealand Journal of Psychiatry, 47(3), 222–34. http://doi.org/10.1177/0004867412468494
Whitehead, M. (1992). The concepts and principles of equity in health. Int J Health Serv, 22(2), 429–445.
World Health Organization. (n.d.). About the Global Burden of Disease (GBD) project. Retrieved April 30, 2015, from http://www.who.int/healthinfo/global_burden_disease/about/en/
Young, T. K. (2004). Population health: concepts and methods. Oxford University Press. Retrieved from http://www.cabdirect.org/abstracts/20043208491.html;jsessionid=408F1B2815A33BC1C58FCBBF67F807B7
Zan, H., and Scharff, R. L. (2014). The Heterogeneity in Financial and Time Burden of Caregiving to Children with Chronic Conditions. Maternal and Child Health Journal, 615–625. http://doi.org/10.1007/s10995-014-1547-3
Zink, A., and Huscher, D. (2004). Longterm studies in rheumatoid arthritis—the German experience. The Journal of Rheumatology. Supplement, 69, 22–26.
