Ten Questions and Answers for Canadian Public Health Practitioners
Preamble
As a result of increased interest and questions about a perceived resurgence or re-emergence of the Influenza A (H1N1) pdm09 this winter, NCCID has undertaken a Rapid Review similar to what had been done for H7N9 and MERS Co-V. However, because this is a review of a previously known pathogen, some of the questions are different than those of the previous two reviews. The purpose of a Rapid Review is to provide a useful analysis and “translation” of current knowledge in a recent or evolving situation. Unlike more in-depth and longer-term collection and analysis of data, Rapid Reviews must use more easily available and incomplete data to facilitate rapid synthesis and dissemination of knowledge that can be used to understand and make decisions for communications and other actions.
For most of our review, we used posted data from the Public Health Agency of Canada’s FluWatch influenza surveillance program, which includes data collected from a network of laboratories, hospitals, doctors’ offices and provincial and territorial ministries. Following a request for input from public health practitioners, including Chief Medical Officers of Health and members of the Public Health Network Communicable and Infectious Diseases Steering Committee, the following ten questions and answers were selected:
Where is Canada in the “epidemic curve” of this year’s influenza season?
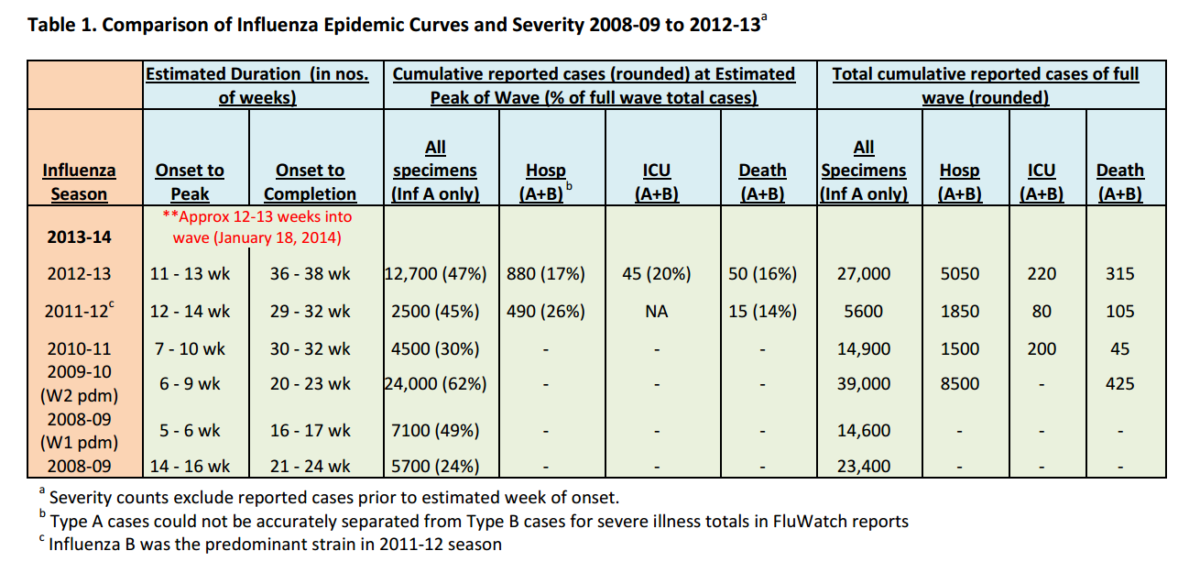
To provide a basis for comparison with previous seasons, NCCID estimated start, peak, and end dates for the influenza seasons of the previous five years using data reported weekly to FluWatch. [i] These estimates are based on lab-confirmed cases of influenza, reported outbreaks and syndromic surveillance of influenza-like illness. Week of onset was defined as the epidemiological week that showed first significant increase in count value, with at least three of the following five weeks being equal or greater values. The reported number of hospitalizations, intensive care unit (ICU) admissions and deaths from participating provinces and territories (even though an underestimate of actual occurrence in Canada), was selected as the best indicator of severity for comparative purposes. Predictions about the totality of severe outcomes for this influenza season cannot be made with confidence at this time, given the unknown magnitude of reporting lags and the uncertain predictability of future wave patterns of any epidemic, especially influenza.
Based on our estimates, the second week of January of 2014 was considered to be half-way through this year’s “flu season”. Therefore, at time of data analysis, we estimate that we are at least two or three weeks after the peak or plateau of activity.
How severe is the current influenza (H1N1) season?
Comment: The duration and pattern of disease frequency for an influenza season can only be known for certain in retrospect, after the wave or waves have occurred. However, based on previous patterns, especially the previous two years, it seemed reasonable to base our comparisons on our best estimate of where we are in the curve. Because we were unable to get data for weekly incidence based on date of onset of symptom, we have used cumulative data. By estimating equivalent time points in the epidemic curve (rather than week numbers of the calendar year), we have attempted to achieve a basis for comparison that is “standardized” by onset dates of the wave. With the exception of the 2008-2009 pandemic season, most seasons of the past few years have had a single wave of case frequency distribution, ranging from four to eight months.
To assess the magnitude of the burden from an infectious disease outbreak or epidemic, the most typical measurements used are attack rates (incidence) severity rates (hospitalization, deaths, premature deaths) and case-fatality proportions.
Anecdotal reports in the media and from some hospital clinicians have described this year’s influenza season as more severe than usual. These reports and the observation from laboratory reports of the return to predominance of the A H1N1 pdm09 strain have raised questions about a return or resurgence of the 2009-2010
pandemic influenza.
Reporting of Influenza-Associated Hospitalizations and Deaths to the Public Health Agency of Canada
Most provinces and territories provide reports to PHAC on hospitalizations and deaths associated with lab-confirmed influenza. For this season and for 2012-2013, these reports have been provided to PHAC by the following eight provinces and territories: Alberta, Saskatchewan, Manitoba, Ontario, Prince Edward Island, Newfoundland and Labrador, Yukon, and Nunavut. In 2011-2012, the provinces of New Brunswick and Nova Scotia also reported. In 2010-2011, all provinces and territories except British Columbia, Quebec and New Brunswick reported. All thirteen provinces and territories reported hospitalizations and deaths in 2009-2010 during the pandemic season.
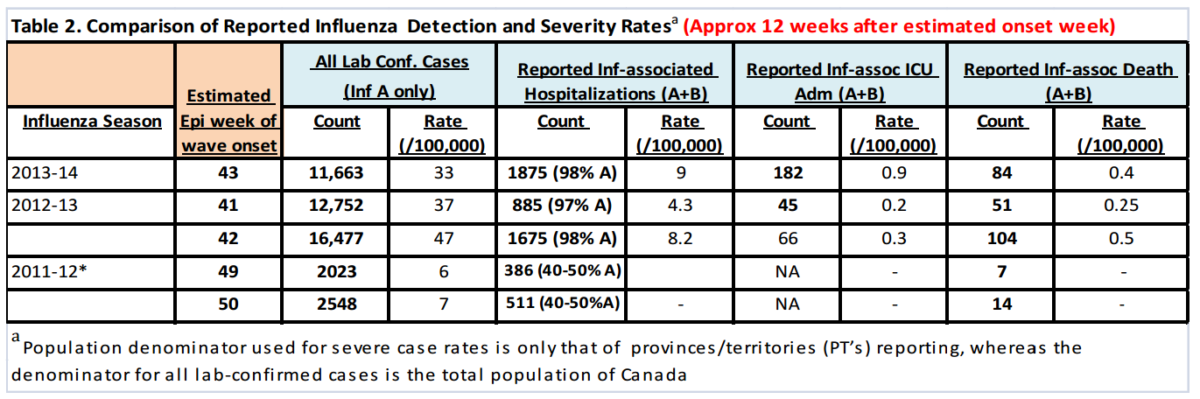
As described earlier, we attempted to gauge this season’s severity by comparing severity measures to those from estimated equivalent time points in the waves from the previous two seasons. With our estimates for week of onset falling within a two week range, we analysed severity data from two consecutive weeks in each of the previous two seasons to compare with this season’s current severity measures (Table 2).
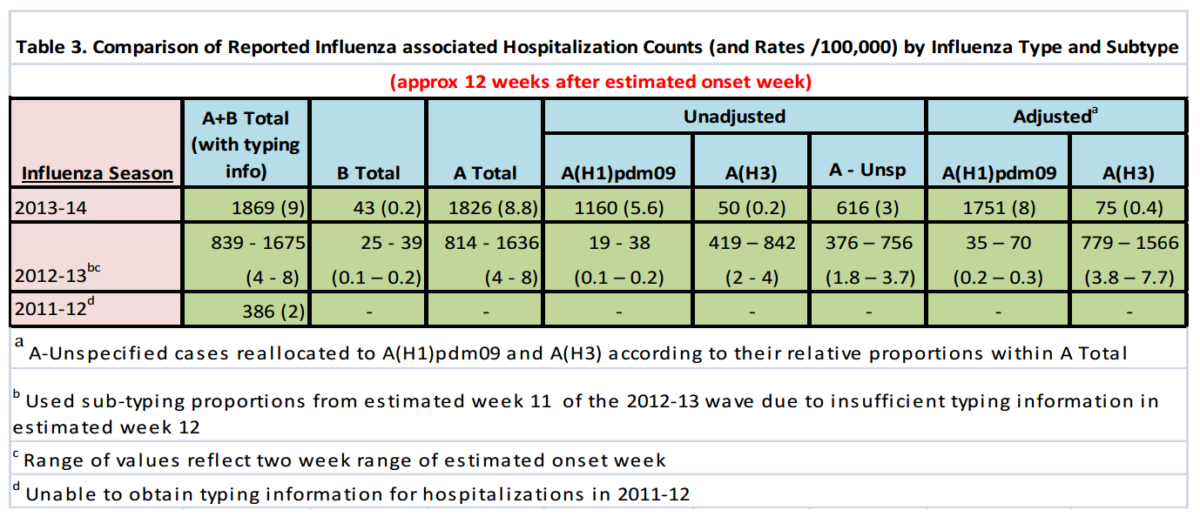
We observed that the overall cumulative rate of hospitalized cases approximately 12 weeks after the estimated week of wave onset for all types of Influenza A were higher than the 2011-12 season and equivalent to or higher than the 2012-13 season. Table 3 below shows the hospitalization rates for known types of influenza and an “adjusted” rate that applies the relative proportions of typed influenza A’s to the un-typed A’s.
As of Jan. 18, 2014, approximately nine in 100,000 Canadians had been hospitalized for all types of Influenza A, more than 90% of which were likely of the H1N1 type. This compares to an approximate rate ranging from four to eight in 100,000 at this point in the wave last season. It will therefore be important to pay close attention to relative severity counts in the coming weeks to ascertain whether this season will indeed prove to be of higher severity than last season. The actual rates of hospitalization associated with influenza at this time are likely significantly higher due to: 1) undiagnosed and / or unreported cases because of lack of testing or timing of testing; and 2) delays in reported cases.
For admissions to intensive care, the reported rates of cases of Influenza at 12 weeks after the estimated onset week have been 0.9 per 100,000 for this season, compared to 0.2-0.3 per 100,000 for last season. As an indicator of very severe disease, these rates are also consistent with a perceived higher rate of severity this season – about five times the rate of last year. From an absolute risk perspective, based on these reported data, at least one in 111,000 Canadians had been admitted to an intensive care unit with lab-confirmed influenza as of January 18, 2014.
Reports of deaths associated with influenza have totaled 84. This is a rate of 0.4 per 100,000 people and compares with 0.25-0.5 per 100,000 last season at this time in the wave. Based on these reported data, the absolute risk of reported death associated with influenza so far this season has been (at least) one in 250,000 people.
All lab-confirmed cases
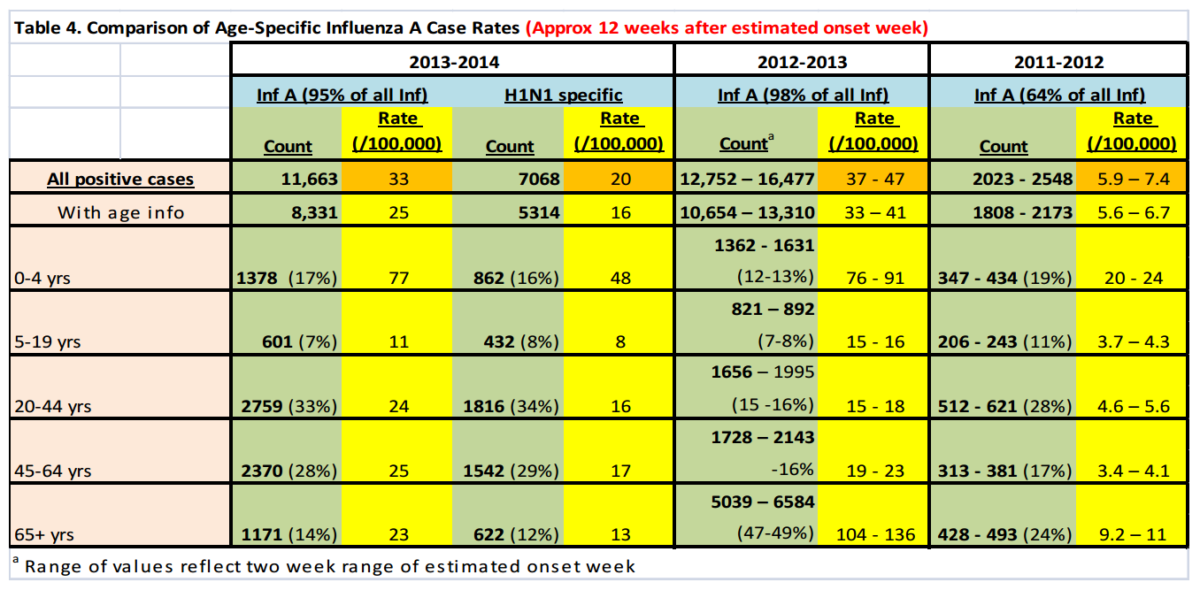
Reported lab-confirmed casesii (Table 4) provide information of age-specific case rates as of January 18, 2014. Although these rates cannot be used to estimate actual attack-rates, they may be useful indicators of the frequency of disease and provide some basis for comparison with previous years. Lab-confirmed case rates of influenza A were generally similar to or less than they were at this time of the wave last year but about five times higher than that of the 2011-2012 season (when Influenza B was more common). Age-specific lab-confirmed case rates have been consistently higher for the youngest (0-4 years) and previously the oldest (>65 years) age categories, but this season has shown a marked reduction in the relative rate among those 65 plus. Differences in age-specific case rates may be due in part to higher rates of testing among certain age groups.
Who has been most at risk for severe disease?
Comment: NCCID was unable to find a standardized set of measurements (or categories) at the international or Canadian national levels to classify absolutely or to compare the relative severity of annual influenza seasons, such as attack rates, severity rates (hospital, intensive care), death rates, premature death rates or potential years of life lost. For this reason, it was decided to make comparisons with recent years, based on available data. However, as has been stated by many chief medical officers of health and others in media reports, the case rates are at best only indicators of disease burden. Variation in testing may be a bigger factor in rate measurement than actual burden of illness. For hospitalized cases and deaths the comparisons may be more meaningful.
However, differences in rates and timeliness of testing and reporting may be very large – even between consecutive years – so any comparisons between rates at any one point in time should be made with caution. Also, without case severity definitions, systematic testing protocols or ways of attributing cause to influenza, our numerators are generated only by those patients that have had a positive lab test result, thereby excluding clinical, probable or epidemiologically linked types of cases and possibly including cases in which influenza may have played a minor, if any, role in the illness or death. In comparison to previous and usual estimates of annual morbidity and mortality from influenza, it is likely that the observed reported rates of deaths and disease – whether mild or severe – are only a small fraction of the actual cases.
This is one explanation why reported numbers are considered just the tip of the iceberg of the estimated 4000-5000 deaths per year in Canada from influenza – extrapolated from other studies and based on estimates of excessive deaths during the influenza season.
Some anecdotal and media reports have indicated that severe or fatal cases of disease or death have been more likely to have a chronic health condition and/or to be unvaccinated than the general population. Based on data collected by PCIRN-SOS as reported in FluWatch report for January 12-18, 2014, “of the 45 ICU admissions with information on influenza vaccination, 32 (71%) reported not having been vaccinated this season”. These immunization rates may not be significantly different from the general population and may be less than among elderly patients who are targeted as higher risk, so these data do not in themselves provide evidence that unvaccinated status has been a risk factor for intensive care. Based on data collected by IMPACT as reported in FluWatch report for January 12-18, 2014, “Among the 33 ICU cases with available data, 25 were reported to have co-morbidities or concurrent infection”. More details are needed to interpret the significance of these observations with respect to risk factors for very severe disease. NCCID compared Canadian age-specific, influenzaassociated hospitalization rates with those described in the Centers for Disease Control and Prevention (CDC) weekly Flu View reportiii and found similar risk groups for severe illness (Table 5). Flu View also noted that, “The most commonly reported underlying medical conditions among adults (based on a 25% subset of cases with complete medical chart abstraction) were obesity, metabolic disorders, cardiovascular disease, and asthma”. In children they were asthma, neurologic disorders, obesity, and cardiovascular disease however approximately 43% of hospitalized children had no identified underlying medical conditions.
Table 5. Influenza-Associated Hospitalizations (Cumulative Rate to Jan. 18, 2014)
| Age Group | US Rate (/100,000) | Canada (/100,000) |
|---|---|---|
| Total | 17 | 9 |
| 0-4 yrs | 27 | 31 |
| 5-17 yrs | 5 | 2.6* |
| 18-49 yrs | 12 | 4.6 |
| 50-64 yrs | 26 | 11 |
| 65+ | 34 | 16 |
COMMENT
It appears evident (and consistent with previous observations for H1N1) that infants and young children, and adults over the age of 50 have been at highest risk for serious illness. One explanation for these observations despite detection rates in adults over 50, could be lower attack rates but more severe disease for those infected. Without clearer definitions of co-morbid conditions and comparative data, it is difficult in real time to quantify attributable risk fractions of risk factors for more severe cases for this season.
What proportion of influenza disease this season has been attributable to H1N1?
As of January 18, 2014, 95% of cumulative influenza virus detections across Canada were influenza A. Among sub-typed influenza A viruses, 97% were A(H1N1)pdm09. Based on testing at the PHAC National Microbiology Laboratory, as reported in FluWatch for January 12-18, 2014 and shown below, the distribution of influenza types and strains (using hemaglutination inhibition (HAI) testing) has shown a significant predominance of the A/California/7/2009(H1N1)pdm09-like virus. The remainder are almost exclusively the two other types and strains whose antigens are contained in the trivalent vaccines developed for the 2013- 2014 influenza season. Antigens in the trivalent vaccine with percentage (in brackets) of lab-confirmed types for this season, as of January 18, 2014 were: A/California/7/2009(H1N1)pdm09-like virus: 354 (83%); A/Texas/50/2012 (H3N2)-like virus: 29 (7%); B/Massachusetts/2/2012-like virus: 38 (9%). Antigens not in the trivalent vaccine with percentage (in brackets) of lab-confirmed types for this season: B/Brisbane/6-/2008-like virus: 5 (1%).
Comment: The high proportion of influenza A that is attributable to H1N1 four years after its pandemic “debut” is consistent with seroprevalence studies last spring which showed significant levels of susceptibility in the population, especially in children under five and amongst younger adults (see question 6).
Is this strain of H1N1 the same as the pandemic strain? To what degree has it drifted?
As of January 18, 2014, of the H1N1 types of Influenza A tested for antibody response using antiserum, 98.8% were antigenically similar to the A/California/7/2009(H1N1)pdm09-like virus.
Comment: It appears that there has been minimal, if any detectable antigenic drift from the zvirus of the 2009-2010 pandemic season.
What is known about the current state of natural or vaccine-induced immunity?
Based on a serosurvey study undertaken in British Columbia prior to this winter season, overall seroprevalence to Influenza H1N1pdm09 approached 50%, but there was significant variation between age groups. Seroprotection was highest (60% or more) in school-aged children and adults over the age of 70, followed by younger adults 20-39 years (45- 50%), middle-aged adults 40-69 years (35-40%); it was lowest in children under five years of age (<20%).
What is known about the efficacy and effectiveness of this year’s influenza vaccine?
Comment: Although these data are limited to one province, they are helpful to interpret and to understand the observed age-specific rates of disease – especially severe disease – this season. Representative and larger samples from across the country and in specific sub-groups of the population could be helpful to anticipate who continues to be at most risk for disease and what severity may be expected in future seasons.
Effectiveness study results for this season’s vaccination were not available as of as of Jan. 18, 2014. However, based on the observation that the three antigens in this season’s vaccine have matched the main circulating influenza viruses, it is reasonable to estimate that previous assessments of efficacy and effectiveness would apply to this year. In general, most published estimates of efficacy and effectiveness against occurrence of lab-confirmed cases of influenza have ranged from 50 to 80% for older children and younger adults, with significantly lower rates for younger children (6-35 months) and older adults (over 50 years).vi These estimates are for all cases of lab-confirmed influenza, most of which were not hospitalized. Effectiveness against hospitalized and/or severe case rates or deaths has been less studied.
Comment: More rapid assessment of vaccine efficacy and effectiveness in the earlier part of the flu season, combined with more rapid and systematic assessment of severity and risk factors should be helpful to determine and adjust vaccine strategies during the flu season, including messaging to health providers and to the public as well as the identification of priority groups for more focused communications and outreach.
What is the potential general benefit of promoting vaccination?
Although it seems reasonable to estimate that January 18, 2014 was at least halfway through this winter’s influenza wave, it is difficult at this time to predict how long this season will last and how much morbidity and mortality may still occur.
Given the observations at this time of higher rates of reported severe disease (see question 2), the significant proportions of the population that were susceptible (see question 6), and the antigen match of this season’s vaccine (see question 7), it would appear to be reasonable to consider continuing to use the vaccine based on its availability.Back to top
If influenza vaccine is still used, which sub-groups of the population should be prioritized, especially in situations of vaccine shortage?
Comment: We do not think that we have sufficient data to make predictions about the anticipated duration of this season, the current state of susceptibility of the population, the remaining proportion of preventable severe illness, and therefore, a quantitative estimate of the most significant benefits of vaccination this late in the season (e.g. prevention of hospitalization or premature deaths).
Based on reported hospitalization rates and estimated seroprotection as of January 18, 2014, it appears that priority for vaccination should be considered for children under five years, adults over 40-50, and anyone with a chronic condition or other disadvantage that may be associated with more severe disease or a decreased ability to access timely and appropriate care for a severe influenza-like-illness.
Comment: It appears that the rates of severe disease have been highest for those age groups that have lowest seroprotecttion (Question 6) and least immune response to vaccination (Question 7) – and possibly natural infection. Therefore, paradoxically, the benefit of vaccination for these age groups and those most at risk may be lowest.
What has NCCID learned from this Rapid Review that could improve the validity, timeliness, and usefulness of surveillance information?
Many answers to the questions of this Rapid Review were provided by the information summarized in FluWatch – a very useful source of relatively current surveillance information. We were however, unable to get some data (current or at all) that would have increased the knowledge available for translation and therefore the usefulness of this Rapid Review.
Comment: Estimating and tracking attack-rates (true incidence) of mild influenza (non-hospitalized cases) require data from systematic and representative sentinel surveillance which include testing protocols and other methods to estimate valid population-based rates. The low reported case counts based on variations of infrequent testing can be misleading for health providers and the public. However, tracking and analyzing the rate of severe cases of influenza and influenza-like-illnesses (ILI) is probably much more important. Protocols for testing hospitalized patients for influenza as well as a wider range of case definitions for clinical cases of hospitalized patients with ILI should provide a more accurate measure of the burden of influenza and provide us with data that could be used to monitor severity systematically and in real-time during the season. In addition, methods to determine the degree to which influenza contributed to severe illness or deaths would also help to determine the burden of illness that is causally associated with influenza.
The following is a list of information and data that we think would be most helpful:
- Case definitions for influenza that are more sensitive and would include clinical and/ or probable cases which take into account the type and timing of testing for influenza;
- Definitions and classification of cases by levels of severity (e.g. duration of hospitalization, need for extra-corporeal membrane oxygenation (ecmo), etc);
- Frequency distributions of cases by week of onset of symptoms;
- Case counts with population denominators collected systematically at a range of representative primary, secondary and tertiary sentinel sites; – Available protocols used for testing and diagnosis to enable valid classifications of cases by likelihood of the contribution of the influenza infection to the severity and outcome of the cases, especially severe illness and death;
- Systematic testing in outpatient and inpatient settings to monitor laboratory characterization of influenza and other respiratory viruses to estimate the proportion of severe cases of influenza-like-illness that may be associated with influenza;
- Systematic and real time surveillance of vaccine effectiveness, especially for severe cases.
i – To estimate weeks of onset, peak and completion of seasonal influenza waves, NCCID looked specifically at weekly reported counts of positive lab specimens, influenza positivity rates, outbreak counts, sentinel surveillance rates of influenza-like-illness, and pharmacy surveillance. Ranges for each wave parameter were generated and a mean or median week was selected for severity analysis.
ii – PHAC’s definition: Clinical illness with laboratory confirmation of infection:
- Isolation of influenza virus from an appropriate clinical specimen OR
- Demonstration of influenza virus antigen in an appropriate clinical specimen OR
- Significant rise (e.g. fourfold or greater) in influenza IgG titre between acute and convalescent sera OR
- Detection of influenza RNA
Ontario includes epidemiologically linked cases in their case definition for influenza.
iii – Centers for Disease Control and Prevention (2014). Influenza Division. 2013-2014 Influenza Season Week 3 ending January 18, 2014. Retrieved from http://www.cdc.gov/flu/weekly/weeklyarchives2013-2014/weekly3.html
iv – Skowronski, PROMED, December 31, 2013.
v – Interim estimates of vaccine effectiveness in 2013-14 season have since been published in Eurosurveillance by Skowronski et al. (2014) http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20690. These estimates appear to be consistent with the previous studies referred to in our report. vi Kissling et al. PLoS ONE. 2011;6(11):e27622; Skowronski et al. J Infect Dis. 2014.; and Skowronski et al. Clin Infect Dis. 2012;55(3):332-42. Full references available on request.
The information used for this Rapid Review was obtained from web-posted reports of the Public Health Agency of Canada and the US Centers for Disease Control and Prevention. Comments are the opinion of Dr. Joel Kettner, Scientific Director of the National Collaborating Centre for Infectious Diseases. We thank Shivoan Balakumar, Salah Mahmud and the staff at FluWatch for their contributions.
