What is POCT and Why It Matters?
Point-of-Care Testing (POCT) involves diagnostic testing carried out in close proximity to the patient, frequently at the patient’s bedside, in a primary care environment or in a community setting, as opposed to being conducted in a centralized clinical laboratory.
POCT are typically administered by individuals outside the traditional laboratory setting, who have undergone appropriate training. This group includes a range of professionals, including but not limited to individuals such as nurses, EMT personnel, dentists, physicians, and pharmacists, along with non-regulated professionals and peers. Additionally, some tests can be conducted by patients themselves for the purpose of managing chronic conditions or diagnosing diseases.
The Benefits of POCT in health care includes:
- Supports Public Health in Emergencies: POCT reduces diagnostic turnaround times, improving patient management and potentially saving lives in emergencies and outbreaks.
- Faster Diagnoses and Treatment: Provides accessible testing for individuals facing barriers to conventional services, enabling timely healthcare access.
- Improved Access in Remote Areas: Benefits remote or resource-limited areas with limited access to centralized labs.
- Supports provision of culturally safe and appropriate testing for First Nation, Inuit and Metis Peoples as part of decentralized, Indigenous-led community based testing initiatives.
- Enables Real-Time Patient Monitoring: Supports quick treatment adjustments and improved outcomes.
- Increased Patient Engagement: Enhances healthcare accessibility and convenience, encouraging greater patient involvement.
- Facilitates Immediate Linkage to Care: Supports rapid diagnosis, enabling immediate linkage to care.
Transforming Diagnostic Practices
in Diverse Settings
Our Network
Understand our organizational structure and how we work alongside health professionals, public health laboratories, national programs, and communities to support POCT’s integration into the healthcare system.
About the POCT Working Group (WG)
The Point of Care Testing (POCT) Working Group (WG) was established in mid-2023, with its first meeting held in May 2023. Under the leadership of the Federal Co-Chairs and with the support of the Jurisdictional Co-Chair, the group is focused on advancing POCT practices across Canada.
Click here to learn more about membership
Membership
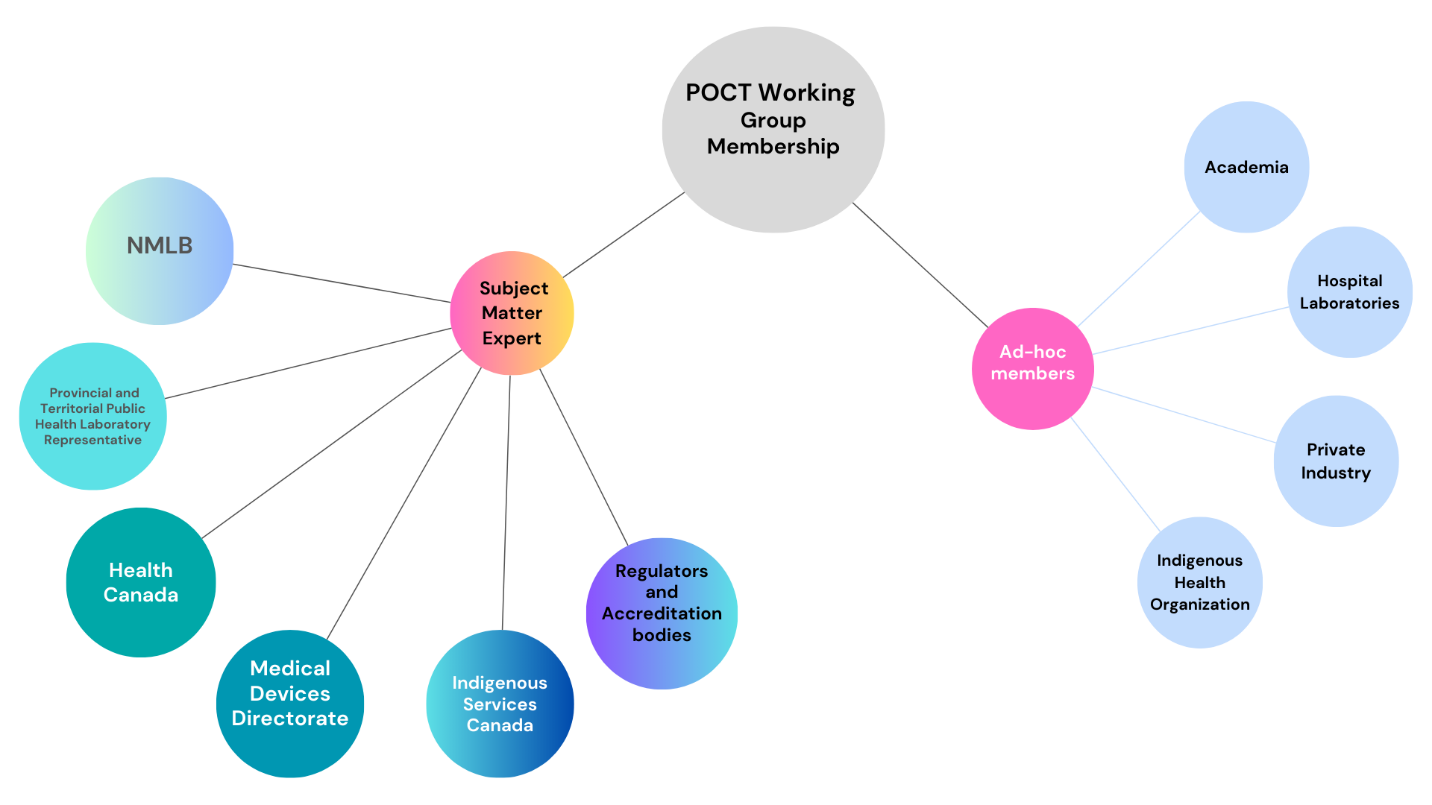
The CPHLN POCT WG is composed of:
- One expert from the National Microbiology Laboratory Branch (NMLB)
- One subject matter expert from each provincial public health laboratory and territorial equivalent.
- Representatives from the Health Canada Medical Devices Directorate, Indigenous Services Canada, and relevant regulatory and accreditation bodies.
Additional Expertise: Specialists from academia, hospital laboratories, private industry, and Indigenous health organizations with relevant expertise may also be invited to participate as ad-hoc, non-voting members. These participants are approved by the working group co-chairs and to contribute to specific mandates or deliverables.
Guidelines and Best Practices
POCT Guideline
Best Practices
- QA Publication from the Australian Group
- Environmental Scan from CADTH
Quality Assurance resources
Regulatory Compliance
Click here to see each province’s unique regulatory standards and quality assurance (QA) guidelines for Point of Care Testing (POCT).
Select a province to learn more:
- Alberta: Learn about Alberta’s standards and quality assurance processes for POCT, regulated by the College of Physicians & Surgeons of Alberta.
- British Columbia: Access British Columbia’s POCT regulatory framework, overseen by the College of Physicians and Surgeons of BC, and Diagnostic Accreditation Program (DAP) including QA standards.
- Manitoba: Discover Manitoba’s POCT guidelines, managed by College of Physicians and Surgeons of Manitoba.
- New Brunswick: Review New Brunswick’s POCT compliance information and QA standards set by the Institute for Quality Management in Healthcare (IQMH).
- Newfoundland & Labrador: Explore Newfoundland & Labrador’s QA and regulatory guidelines for POCT, regulated by the Institute for Quality Management in Healthcare (IQMH).
- Nova Scotia: Find information on POCT standards and QA protocols in Nova Scotia, governed by IQMH; Accreditation Canada.
- Ontario: Learn about Ontario’s comprehensive (POCT/Laboratory) regulatory standards, managed by the Ontario Medical Association through IQMH and associated QA guidelines.
- Prince Edward Island: Review Prince Edward Island’s guidelines and QA practices for POCT, regulated by Accreditation Canada.
- Quebec: Access Quebec’s unique POCT framework, governed by the Canadian Standards Association and associated quality assurance standards.
- Saskatchewan: Discover Saskatchewan’s POCT standards, including QA protocols overseen by the College of Physicians and Surgeons of Saskatchewan.
- Yukon, Northwest Territories & Nunavut: Learn about the POCT practices and compliance guidelines across Canada’s territories, supported by federal and territorial health departments. (In the latest CADTH Environmental Scan, no provincial regulatory standards specific to Point of Care Testing (POCT) were identified).
POCT Programs and Services
Overview of POCT Programs
Explore the diverse POCT programs offered, including rapid diagnostics, infectious disease detection, and more.
Implementation Steps
Please enter the password to view this block:
Implementation Support
Click here to learn how the POCT Committee supports the implementation of POCT in healthcare settings through training, quality assurance, and best practices.
Contact Organization: If you’re interested in starting a project, please reach out to your provincial organization for guidance.
Checklist to come……..
Quality Assurance Considerations
Information on assurance consideration.
Quality Control
Quality Assurance Standards
Information on the QA measures in place to ensure accuracy and reliability in POCT results.
Proficiency Testing
Information on proficiency testing programs.
Training and Certification
Information on training and certification.
Information on Available Training Programs
Details on enrollment process.
Educational Resources
Tools and Templates
Download helpful tools, checklists, and templates to aid in the effective implementation and management of POCT.
- Canada’s Drug Agency (CADTH) – Point-of-Care Testing Environmental Scan
Accreditation
Information on accreditation.
Validated POCT Devices
Validation Process
For the most current information on approved point-of-care testing devices, visit Health Canada’s Medical Device Active Licence Listing (MDALL). Note that some devices may have had their approval rescinded after initial authorization, so it is recommended to verify the device’s current status on the MDALL.

Case Studies
Challenges Experienced Across Different Contexts and proposed solutions
Discover how these challenges are being addressed through innovative solutions and collaborative efforts.
Improving Community Health: A Case Story on Group A Streptococcal Screening in Quebec
Group A Streptococcus (GAS), a leading cause of pharyngitis and tonsillitis, impacts thousands of Quebecers annually. Responsible for 20–30% of sore throat cases in children and 5–15% in adults, GAS infections often lead to emergency room visits, particularly among children. Recognizing the need for accessible and efficient healthcare solutions, Santé Québec reimagined the role of Points de Service Locaux (PSL)—community-based service centers established during the COVID-19 pandemic—to include GAS screening services. This innovative approach aimed to make healthcare more accessible by bringing screening and treatment closer to patients’ homes, reducing the strain on emergency departments, and ensuring timely care. The benefits of this model included:
- Convenient Appointment Scheduling: Patients could easily book appointments online through Clic Santé, streamlining the process and minimizing delays.
- Accessibility in Community Settings: Points de service locaux (PSLs) were strategically located in familiar, convenient locations like shopping centers, making care more accessible.
- Fast and Efficient Testing: Nurses performed Point-of-Care Testing (POCT) for Group A Streptococcus (GAS) on-site, delivering results within minutes.
- Immediate Access to Treatment: Positive cases were provided with prescriptions on the spot, enabling same-day treatment and reducing the risk of complications.
- Equitable Care for All: By offering the service free of charge, barriers related to cost were eliminated, ensuring equal access to timely care for everyone.
Initially, the program faced significant challenges, including a shortage of fully licensed staff, inconsistent testing methods across regions, and limited awareness among the public and healthcare providers. However, these hurdles are being addressed through three innovative solutions:
- Staffing workflows were restructured to include registered nurses or fully licensed retired nurses,
- Standardized protocols will be introduced by the Institut national d’excellence en santé et service sociaux (INESSS) to ensure consistency in testing and prescriptions,
- A targeted awareness campaign, supported by regional collaboration, will effectively close communication gaps and boost program adoption.
Lessons Learned and looking Ahead:
- Importance of pre-planning and establishing clear protocols before launching new programs.
- Collaboration and effective communication with stakeholders are critical to success.
- By expanding PSLs to include GAS screening, Santé Québec has enhanced healthcare accessibility and set a benchmark for leveraging point-of-care testing (POCT) to improve public health outcomes.
Key Initiatives
Collaborative Efforts Focused on POCT Best Practices
- Frontline Perspectives on POCT: We are conducting a survey to gather insights from frontline workers on their experiences with POCT.
Please enter the password to view this block:
For further information please contact the CPHLN Secretariat at CPHLN-RLSPC@phac-aspc.gc.ca
